Page 148 - Essentials of physical chemistry
P. 148
110 Essentials of Physical Chemistry
TABLE 6.1
Vapor Pressures of Selected Liquids at Various Temperatures (8C)
Vapor Pressure CCl 4 C 2 H 5 OH CH 3 COOH H 2 O C 6 H 6
1Pa 79.4 (s) 73 e 42.8 (s) 60.7 (s) —
10 Pa 70.8 (s) 56 e 26.7 (s) 42.2 (s) —
100 Pa 53.5 (s) 34 e 8 (s) 20.3 (s) 40 (s)
1 kPa 24.4 (s) 7 e 14.2 (s) 7.0 15.1 (s)
10 kPa 15.8 29.2 55.9 45.8 20.0
100 kPa (1 bar) 76.2 78.0 117.5 99.6 79.7
Source: Lide, D.R., CRC Handbook of Chemistry and Physics, 90th Edn., CRC Press, Boca
Raton, FL, 2009–2010, pp. 6–72. With permission.
(s) ¼ solid, e ¼ extrapolated estimate.
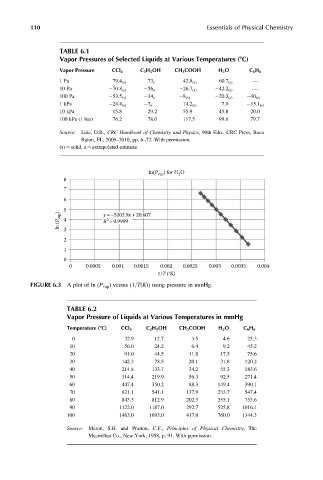
ln(P
2
vap ) for H O
8
7
6
5
ln (P vap ) 4 y =–5202.9x +20.607
2
R =0.9999
3
2
1
0
0 0.0005 0.001 0.0015 0.002 0.0025 0.003 0.0035 0.004
1/T (°K)
FIGURE 6.3 A plot of ln (P vap ) versus (1=T(K)) using pressure in mmHg.
TABLE 6.2
Vapor Pressure of Liquids at Various Temperatures in mmHg
Temperature (8C) CCl 4 C 2 H 5 OH CH 3 COOH H 2 O C 6 H 6
0 32.9 12.7 3.5 4.6 25.3
10 56.0 24.2 6.4 9.2 45.2
20 91.0 44.5 11.8 17.5 75.6
30 142.3 78.5 20.1 31.8 120.2
40 214.8 133.7 34.2 55.3 183.6
50 314.4 219.9 56.3 92.5 271.4
60 447.4 350.2 88.3 149.4 390.1
70 621.1 541.1 137.9 233.7 547.4
80 843.3 812.9 202.3 355.1 753.6
90 1122.0 1187.0 292.7 525.8 1016.1
100 1463.0 1693.0 417.0 760.0 1344.3
Source: Maron, S.H. and Prutton, C.F., Principles of Physical Chemistry, The
Macmillan Co., New York, 1958, p. 91. With permission.