Page 200 - Essentials of physical chemistry
P. 200
162 Essentials of Physical Chemistry
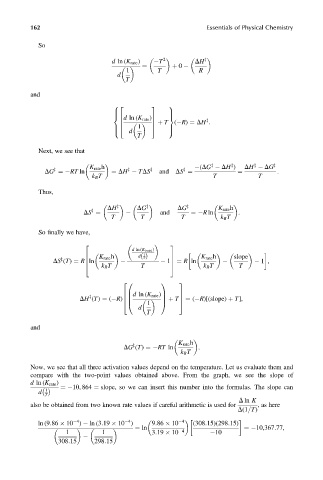
So
2
d ln (K rate ) T DH z
¼ þ 0
1 T R
d
T
and
8 2 3 9
> >
> >
< =
6 d ln (K rate )7
z
6 7 þ T ( R) ¼ DH :
4 1 5
> >
> >
: d ;
T
Next, we see that
K rate h (DG DH ) DH DG z
z
z
z
DG ¼ RT ln ¼ DH TDS z and DS ¼ ¼ :
z
z
z
k B T T T
Thus,
DH z DG z DG z K rate h
and ¼ R ln :
z
DS ¼
T T T k B T
So finally we have,
2 3
d ln (K (rate) )
K rate h 1 K rate h slope
T
6 dðÞ 7
DS (T) ¼ R ln 1 ¼ R ln 1 ,
z
7
6
k B T T k B T T
4 5
2 0 1 3
6 B d ln (K rate )C 7
DH (T) ¼ ( R) 6 B C þ T 7 ¼ ( R)[(slope) þ T],
z
1
4 @ A 5
d
T
and
K rate h
DG (T) ¼ RT ln :
z
k B T
Now, we see that all three activation values depend on the temperature. Let us evaluate them and
compare with the two-point values obtained above. From the graph, we see the slope of
d ln (K rate )
¼ 10, 864 ¼ slope, so we can insert this number into the formulas. The slope can
d 1
T
D ln K
also be obtained from two known rate values if careful arithmetic is used for ,ashere
D(1=T)
4
4
ln (9:86 10 ) ln (3:19 10 ) 9:86 10 4 (308:15)(298:15)
¼ ln ¼ 10,367:77,
4
1 1 3:19 10 10
308:15 298:15