Page 211 - Essentials of physical chemistry
P. 211
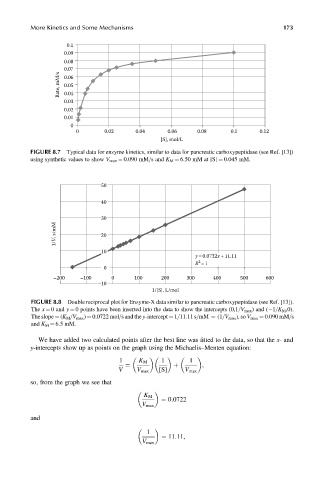
More Kinetics and Some Mechanisms 173
0.1
0.09
0.08
Rate, mM/s 0.07
0.06
0.05
0.04
0.03
0.02
0.01
0
0 0.02 0.04 0.06 0.08 0.1 0.12
[S], mol/L
FIGURE 8.7 Typical data for enzyme kinetics, similar to data for pancreatic carboxypeptidase (see Ref. [13])
using synthetic values to show V max ¼ 0.090 mM=s and K M ¼ 6.50 mM at [S] ¼ 0.045 mM.
50
40
30
1/V, s/mM 20
10
y =0.0722x +11.11
2
R =1
0
–200 –100 0 100 200 300 400 500 600
–10
1/[S], L/mol
FIGURE 8.8 Double reciprocal plot for Enzyme-X data similar to pancreatic carboxypeptidase (see Ref. [13]).
The x ¼ 0 and y ¼ 0 points have been inserted into the data to show the intercepts (0,1=V max ) and ( 1=K M ,0).
The slope ¼ (K M =V max ) ¼ 0.0722 mol=s and the y-intercept ¼ 1=11.11 s=mM ¼ (1=V max ), so V max ¼ 0.090 mM=s
and K M ¼ 6.5 mM.
We have added two calculated points after the best line was fitted to the data, so that the x- and
y-intercepts show up as points on the graph using the Michaelis–Menten equation:
1 K M 1 1
,
þ
V V max [S] V max
¼
so, from the graph we see that
K M
¼ 0:0722
V max
and
1
¼ 11:11,
V max