Page 212 - Essentials of physical chemistry
P. 212
174 Essentials of Physical Chemistry
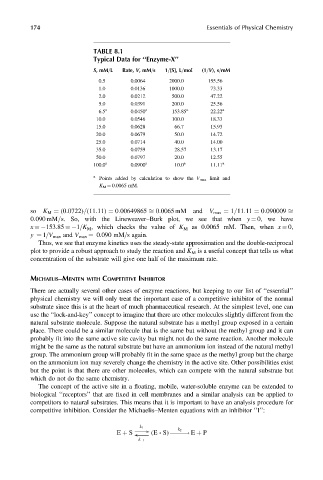
TABLE 8.1
Typical Data for ‘‘Enzyme-X’’
S,mM=L Rate, V,mM=s 1=[S], L=mol (1=V), s=mM
0.5 0.0064 2000.0 155.56
1.0 0.0136 1000.0 73.33
2.0 0.0212 500.0 47.22
5.0 0.0391 200.0 25.56
6.5 a 0.0450 a 153.85 a 22.22 a
10.0 0.0546 100.0 18.33
15.0 0.0628 66.7 15.93
20.0 0.0679 50.0 14.72
25.0 0.0714 40.0 14.00
35.0 0.0759 28.57 13.17
50.0 0.0797 20.0 12.55
100.0 a 0.0900 a 10.0 a 11.11 a
a
Points added by calculation to show the V max limit and
K M ¼ 0.0065 mM.
so K M ¼ (0:0722)=(11:11) ¼ 0:00649865 ffi 0:0065 mM and V max ¼ 1=11:11 ¼ 0:090009 ffi
0:090 mM=s. So, with the Lineweaver–Burk plot, we see that when y ¼ 0, we have
x ¼ 153.85 ¼ 1=K M , which checks the value of K M as 0.0065 mM. Then, when x ¼ 0,
y ¼ 1=V max and V max ¼ 0.090 mM=s again.
Thus, we see that enzyme kinetics uses the steady-state approximation and the double-reciprocal
plot to provide a robust approach to study the reaction and K M is a useful concept that tells us what
concentration of the substrate will give one half of the maximum rate.
MICHAELIS–MENTEN WITH COMPETITIVE INHIBITOR
There are actually several other cases of enzyme reactions, but keeping to our list of ‘‘essential’’
physical chemistry we will only treat the important case of a competitive inhibitor of the normal
substrate since this is at the heart of much pharmaceutical research. At the simplest level, one can
use the ‘‘lock-and-key’’ concept to imagine that there are other molecules slightly different from the
natural substrate molecule. Suppose the natural substrate has a methyl group exposed in a certain
place. There could be a similar molecule that is the same but without the methyl group and it can
probably fit into the same active site cavity but might not do the same reaction. Another molecule
might be the same as the natural substrate but have an ammonium ion instead of the natural methyl
group. The ammonium group will probably fit in the same space as the methyl group but the charge
on the ammonium ion may severely change the chemistry in the active site. Other possibilities exist
but the point is that there are other molecules, which can compete with the natural substrate but
which do not do the same chemistry.
The concept of the active site in a floating, mobile, water-soluble enzyme can be extended to
biological ‘‘receptors’’ that are fixed in cell membranes and a similar analysis can be applied to
competitors to natural substrates. This means that it is important to have an analysis procedure for
competitive inhibition. Consider the Michaelis–Menten equations with an inhibitor ‘‘I’’:
k 1
k 2
E þ S ! (E S) ! E þ P
k 1