Page 214 - Essentials of physical chemistry
P. 214
176 Essentials of Physical Chemistry
No inhibitor V max
Competitive
V I
inhibition
V max
1/2 V max
Noncompetitive
inhibition
1/2 V max
[S]
K M
K M
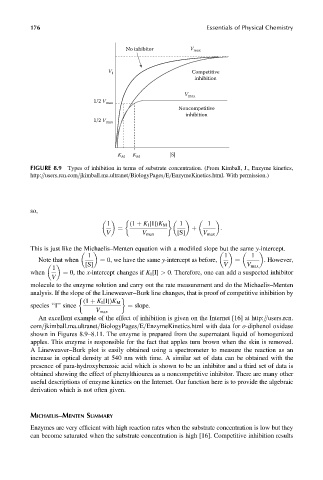
FIGURE 8.9 Types of inhibition in terms of substrate concentration. (From Kimball, J., Enzyme kinetics,
http:==users.rcn.com=jkimball.ma.ultranet=BiologyPages=E=EnzymeKinetics.html. With permission.)
so,
1 (1 þ K I [I])K M 1 1
:
¼ þ
V V max [S] V max
This is just like the Michaelis–Menten equation with a modified slope but the same y-intercept.
1 1 1
Note that when ¼ 0, we have the same y-intercept as before, ¼ . However,
[S] V V max
1
when ¼ 0, the x-intercept changes if K I [I] > 0. Therefore, one can add a suspected inhibitor
V
molecule to the enzyme solution and carry out the rate measurement and do the Michaelis–Menten
analysis. If the slope of the Lineweaver–Burk line changes, that is proof of competitive inhibition by
(1 þ K I [I])K M
species ‘‘I’’ since ¼ slope.
V max
An excellent example of the effect of inhibition is given on the Internet [16] at http:==users.rcn.
com=jkimball.ma.ultranet=BiologyPages=E=EnzymeKinetics.html with data for o-diphenol oxidase
shown in Figures 8.9–8.11. The enzyme is prepared from the supernatant liquid of homogenized
apples. This enzyme is responsible for the fact that apples turn brown when the skin is removed.
A Lineweaver–Burk plot is easily obtained using a spectrometer to measure the reaction as an
increase in optical density at 540 nm with time. A similar set of data can be obtained with the
presence of para-hydroxybenzoic acid which is shown to be an inhibitor and a third set of data is
obtained showing the effect of phenylthiourea as a noncompetitive inhibitor. There are many other
useful descriptions of enzyme kinetics on the Internet. Our function here is to provide the algebraic
derivation which is not often given.
MICHAELIS–MENTEN SUMMARY
Enzymes are very efficient with high reaction rates when the substrate concentration is low but they
can become saturated when the substrate concentration is high [16]. Competitive inhibition results