Page 215 - Essentials of physical chemistry
P. 215
More Kinetics and Some Mechanisms 177
Noncompetitive
Competitive
inhibition
inhibition
1
V I
K M
Slope = V max
1
V max
1
–1
[S]
K M
FIGURE 8.10 Lineweaver–Burk plots of inhibition.
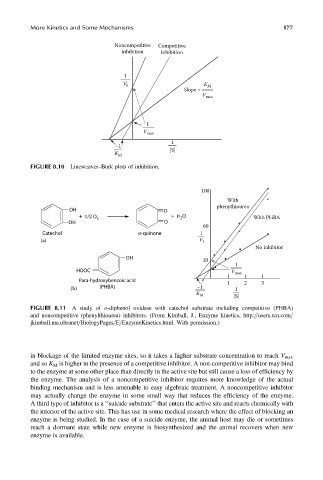
100
With
OH O phenythiourea
+ 1/2 O + H O
2 2 With PHBA
OH O
60
Catechol o-quinone 1
(a) V I
No inhibitor
OH
20
1
HOOC V max
Para-hydroxybenzoic acid
(PHBA) 1 2 3
–1
(b)
1
K M
[S]
FIGURE 8.11 A study of o-diphenol oxidase with catechol substrate including competitive (PHBA)
and noncompetitive (phenylthiourea) inhibitors. (From Kimball, J., Enzyme kinetics, http:==users.rcn.com=
jkimball.ma.ultranet=BiologyPages=E=EnzymeKinetics.html. With permission.)
in blockage of the limited enzyme sites, so it takes a higher substrate concentration to reach V max
and so K M is higher in the presence of a competitive inhibitor. A non-competitive inhibitor may bind
to the enzyme at some other place than directly in the active site but still cause a loss of efficiency by
the enzyme. The analysis of a noncompetitive inhibitor requires more knowledge of the actual
binding mechanism and is less amenable to easy algebraic treatment. A noncompetitive inhibitor
may actually change the enzyme in some small way that reduces the efficiency of the enzyme.
A third type of inhibitor is a ‘‘suicide substrate’’ that enters the active site and reacts chemically with
the interior of the active site. This has use in some medical research where the effect of blocking an
enzyme is being studied. In the case of a suicide enzyme, the animal host may die or sometimes
reach a dormant state while new enzyme is biosynthesized and the animal recovers when new
enzyme is available.