Page 207 - Facility Piping Systems Handbook for Industrial, Commercial, and Healthcare Facilities
P. 207
WATER TREATMENT AND PURIFICATION
WATER TREATMENT AND PURIFICATION 4.43
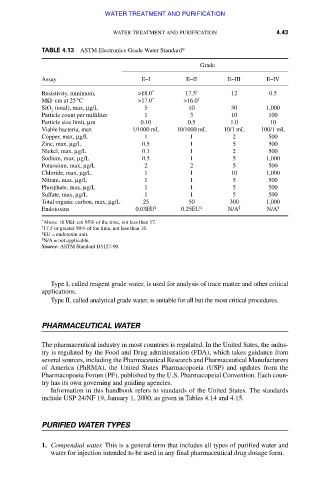
TABLE 4.13 ASTM Electronics-Grade Water Standard*
Grade
Assay E–I E–II E–III E–IV
Resistivity, minimum, >18.0 * 17.5 † 12 0.5
MΩ⋅ cm at 25 °C >17.0 * >16.0 †
SiO (total), max, μg/L 5 10 50 1,000
2
Particle count per milliliter 1 3 10 100
Particle size limit, μm 0.10 0.5 1.0 10
Viable bacteria, max 1/1000 mL 10/1000 mL 10/1 mL 100/1 mL
Copper, max, μg/L 1 1 2 500
Zinc, max, μg/L 0.5 1 5 500
Nickel, max, μg/L 0.1 1 2 500
Sodium, max, μg/L 0.5 1 5 1,000
Potassium, max, μg/L 2 2 5 500
Chloride, max, μg/L 1 1 10 1,000
Nitrate, max, μg/L 1 1 5 500
Phosphate, max, μg/L 1 1 5 500
Sulfate, max, μg/L 1 1 5 500
Total organic carbon, max, μg/L 25 50 300 1,000
Endotoxins 0.03EU ‡ 0.25EU ‡ N/A § N/A §
*
Above 18 MΩ . cm 95% of the time, not less than 17.
†
17.5 or greater 90% of the time, not less than 16.
‡
EU = endotoxin unit.
§
N/A = not applicable.
Source: ASTM Standard D5127-90.
Type I, called reagent grade water, is used for analysis of trace matter and other critical
applications.
Type II, called analytical grade water, is suitable for all but the most critical procedures.
PHARMACEUTICAL WATER
The pharmaceutical industry in most countries is regulated. In the United Sates, the indus-
try is regulated by the Food and Drug administration (FDA), which takes guidance from
several sources, including the Pharmaceutical Research and Pharmaceutical Manufacturers
of America (PhRMA), the United States Pharmacopoeia (USP) and updates from the
Pharmacopoeia Forum (PF), published by the U.S. Pharmacopeial Convention. Each coun-
try has its own governing and guiding agencies.
Information in this handbook refers to standards of the United States. The standards
include USP 24/NF 19, January 1, 2000, as given in Tables 4.14 and 4.15.
PURIFIED WATER TYPES
1. Compendial water. This is a general term that includes all types of purified water and
water for injection intended to be used in any final pharmaceutical drug dosage form.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.accessengineeringlibrary.com)
Copyright © 2009 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.