Page 29 - Fiber Fracture
P. 29
14 K.K. Chawla
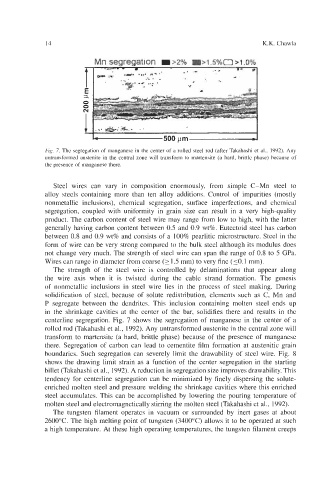
Fig. 7. The segregation of manganese in the center of a rolled steel rod (after Takahashi et al., 1992). Any
untransformed austenite in the central zone will transform to martensite (a hard, brittle phase) because of
the presence of manganese there.
Steel wires can vary in composition enormously, from simple C-Mn steel to
alloy steels containing more than ten alloy additions. Control of impurities (mostly
nonmetallic inclusions), chemical segregation, surface imperfections, and chemical
segregation, coupled with uniformity in grain size can result in a very high-quality
product. The carbon content of steel wire may range from low to high, with the latter
generally having carbon content between 0.5 and 0.9 wt%. Eutectoid steel has carbon
between 0.8 and 0.9 wt% and consists of a 100% pearlitic microstructure. Steel in the
form of wire can be very strong compared to the bulk steel although its modulus does
not change very much. The strength of steel wire can span the range of 0.8 to 5 GPa.
Wires can range in diameter from coarse (2 1.5 mm) to very fine (10.1 mm).
The strength of the steel wire is controlled by delaminations that appear along
the wire axis when it is twisted during the cable strand formation. The genesis
of nonmetallic inclusions in steel wire lies in the process of steel making. During
solidification of steel, because of solute redistribution, elements such as C, Mn and
P segregate between the dendrites. This inclusion containing molten steel ends up
in the shrinkage cavities at the center of the bar, solidifies there and results in the
centerline segregation. Fig. 7 shows the segregation of manganese in the center of a
rolled rod (Takahashi et al., 1992). Any untransformed austenite in the central zone will
transform to martensite (a hard, brittle phase) because of the presence of manganese
there. Segregation of carbon can lead to cementite film formation at austenitic grain
boundaries. Such segregation can severely limit the drawability of steel wire. Fig. 8
shows the drawing limit strain as a function of the center segregation in the starting
billet (Takahashi et al., 1992). A reduction in segregation size improves drawability. This
tendency for centerline segregation can be minimized by finely dispersing the solute-
enriched molten steel and pressure welding the shrinkage cavities where this enriched
steel accumulates. This can be accomplished by lowering the pouring temperature of
molten steel and electromagnetically stirring the molten steel (Takahashi et al., 1992).
The tungsten filament operates in vacuum or surrounded by inert gases at about
2600°C. The high melting point of tungsten (3400°C) allows it to be operated at such
a high temperature. At these high operating temperatures, the tungsten filament creeps