Page 789 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 789
744 Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological
0.30 net cell yields decline with the residence time in the reactor
0.25 Carbohydrates due to the endogenous decay of organisms.
Y (kg VSS/kg BOD L ) 0.20 As with aerobic reactions, Monod kinetics may apply to
Kinetics
23.4.3.2
0.15
anaerobic reactions, as reviewed in Section 22.5. The
Monod equation is restated,
0.10
0.05 Proteins S
Fatty acids m ¼ ^m (22:31)
0.00 K s þ S
0 5 10 15 20 25 30
SRT (day) The kinetic equations apply to both stages of the anaerobic
process, i.e., acid formation or methane formation, or empir-
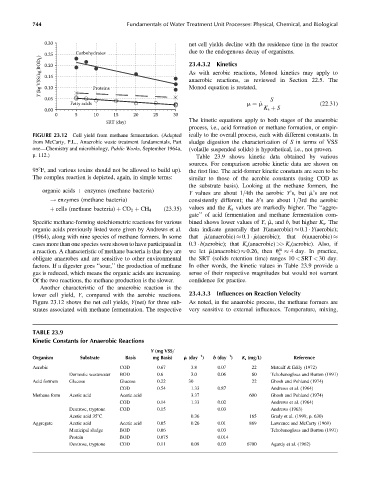
FIGURE 23.12 Cell yield from methane fermentation. (Adapted ically to the overall process, each with different constants. In
from McCarty, P.L., Anaerobic waste treatment fundamentals, Part sludge digestion the characterization of S in terms of VSS
one—Chemistry and microbiology, Public Works, September 1964a, (volatile suspended solids) is hypothetical, i.e., not proven.
p. 112.) Table 23.9 shows kinetic data obtained by various
sources. For comparison aerobic kinetic data are shown on
958F, and various toxins should not be allowed to build up). the first line. The acid-former kinetic constants are seen to be
The complex reaction is depicted, again, in simple terms: similar to those of the aerobic constants (using COD as
the substrate basis). Looking at the methane formers, the
organic acids þ enzymes (methane bacteria)
Y values are about 1=4th the aerobic Y’s, but ^m’s are not
! enzymes (methane bacteria) consistently different; the b’s are about 1=3rd the aerobic
(23:35) values and the K s values are markedly higher. The ‘‘aggre-
þ cells (methane bacteria) þ CO 2 þ CH 4
gate’’ of acid fermentation and methane fermentation com-
Specific methane-forming stoichiometric reactions for various bined shows lower values of Y, ^m, and b, but higher K s . The
organic acids previously listed were given by Andrews et al. data indicate generally that Y(anaerobic) 0.1 Y(aerobic);
(1964), along with nine species of methane formers. In some that ^ m(anaerobic) 0.1 ^m(aerobic); that b(anaerobic)
cases more than one species were shown to have participated in 0.3 b(aerobic); that K s (anaerobic) >> K s (aerobic). Also, if
m
a reaction. A characteristic of methane bacteria is that they are we let ^m(anaerobic) 0.26, then u 4 day. In practice,
c
obligate anaerobes and are sensitive to other environmental the SRT (solids retention time) ranges 10 < SRT < 30 day.
factors. If a digester goes ‘‘sour,’’ the production of methane In other words, the kinetic values in Table 23.9 provide a
gas is reduced, which means the organic acids are increasing. sense of their respective magnitudes but would not warrant
Of the two reactions, the methane production is the slower. confidence for practice.
Another characteristic of the anaerobic reaction is the
lower cell yield, Y, compared with the aerobic reactions. 23.4.3.3 Influences on Reaction Velocity
Figure 23.12 shows the net cell yields, Y(net) for three sub- As noted, in the anaerobic process, the methane formers are
strates associated with methane fermentation. The respective very sensitive to external influences. Temperature, mixing,
TABLE 23.9
Kinetic Constants for Anaerobic Reactions
Y (mg VSS=
1
1
Organism Substrate Basis mg Basis) ^ m (day ) b (day ) K s (mg=L) Reference
Aerobic COD 0.67 3.8 0.07 22 Metcalf & Eddy (1972)
Domestic wastewater BOD 0.6 3.0 0.06 60 Tchobanoglous and Burton (1991)
Acid formers Glucose Glucose 0.22 30 22 Ghosh and Pohland (1974)
COD 0.54 1.33 0.87 Andrews et al. (1964)
Methane form Acetic acid Acetic acid 3.37 600 Ghosh and Pohland (1974)
COD 0.14 1.33 0.02 Andrews et al. (1964)
Dextrose, tryptone COD 0.15 0.03 Andrews (1963)
Acetic acid 358C 0.36 165 Grady et al. (1999, p. 630)
Aggregate Acetic acid Acetic acid 0.05 0.26 0.01 869 Lawrence and McCarty (1969)
Municipal sludge BOD 0.06 0.03 Tchobanoglous and Burton (1991)
Protein BOD 0.075 0.014
Dextrose, tryptone COD 0.11 0.09 0.03 6700 Agardy et al. (1962)