Page 906 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 906
Appendix H: Dissolved Gases 861
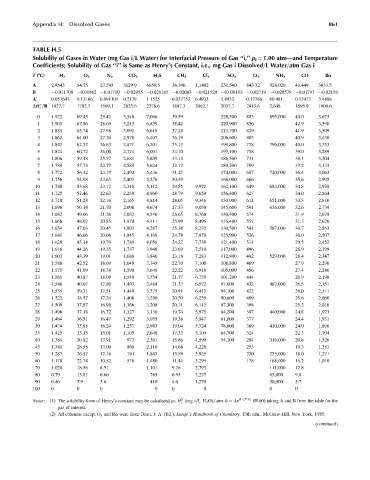
TABLE H.5
Solubility of Gases in Water (mg Gas i=L Water) for Interfacial Pressure of Gas ‘‘i,’’ p i ¼ 1.00 atm—and Temperature
Coefficients; Solubility of Gas ‘‘i’’ is Same as Henry’s Constant, i.e., mg Gas i Dissolved=L Water=atm Gas i
T (8C) H 2 O 2 N 2 CO 2 H 2 S CH 4 Cl 2 SO 2 O 3 NH 3 CO Rn
A 2.4543 64.75 27.593 3129.9 6659.5 36.396 1,1402 231,540 843.32 928,020 41.449 3633.5
B 0.011708 0.01862 0.01710 0.02955 0.026105 0.02063 0.021520 0.038103 0.02719 0.028579 0.01747 0.02156
A 0 0.057647 0.13166 0.091101 0.2179 1.1525 0.037757 6.4933 3.4932 0.12786 60.481 0.12413 3.4488
DH =R 1027.7 1702.3 1569.1 2625.6 2378.6 1887.3 2062.1 3037.7 2413.6 2,648 1595.9 1908.6
i
0 1.922 69.45 29.42 3,346 7,066 39.59 228,300 883 895,000 44.0 3,673
1 1.901 67.56 28.69 3,213 6,839 38.42 220,900 856 42.9 3,590
2 1.881 65.74 27.98 3,091 6,619 37.28 213,700 829 41.9 3,509
3 1.862 64.00 27.30 2,978 6,407 36.19 206,600 803 40.9 3,430
4 1.843 62.32 26.63 2,871 6,201 35.13 199,800 778 796,000 40.0 3,353
5 1.824 60.72 26.00 2,774 6,001 34.10 193,100 758 39.0 3,289
6 1.806 59.18 25.37 2,681 5,809 33.12 186,500 731 38.1 3,204
7 1.789 57.73 24.77 2,589 5,624 32.17 180,200 709 37.2 3,133
8 1.772 56.32 24.19 2,492 5,446 31.27 174,000 687 720,000 36.4 3,063
9 1.756 54.98 23.65 2,403 5,276 30.39 168,000 666 35.6 2,995
10 1.740 53.68 23.12 2,318 5,112 29.55 9,972 162,100 649 684,000 34.8 2,938
11 1.725 52.46 22.63 2,239 4,960 28.79 9,654 156,400 627 34.0 2,864
12 1.710 51.28 22.16 2,165 4,814 28.05 9,346 150,900 611 651,000 33.3 2,810
13 1.696 50.14 21.70 2,098 4,674 27.33 9,050 145,600 591 636,000 32.6 2,739
14 1.682 49.06 21.26 2,032 4,540 26.65 8,768 140,400 574 31.9 2,679
15 1.668 48.02 20.85 1,970 4,411 25.99 8,495 135,400 557 31.3 2,620
16 1.654 47.03 20.45 1,903 4,287 25.38 8,232 130,500 541 587,000 30.7 2,563
17 1.641 46.06 20.06 1,845 4,169 24.78 7,979 125,900 526 30.0 2,507
18 1.628 45.14 19.70 1,789 4,056 24.22 7,738 121,400 511 29.5 2,452
19 1.616 44.26 19.35 1,737 3,948 23.69 7,510 117,000 496 28.9 2,399
20 1.603 43.39 19.01 1,688 3,846 23.18 7,283 112,800 482 529,000 28.4 2,347
21 1.588 42.52 18.69 1,640 3,745 22.70 7,100 108,800 469 27.9 2,296
22 1.575 41.69 18.38 1,590 3,648 22.22 6,918 105,000 456 27.4 2,246
23 1.561 40.87 18.09 1,540 3,554 21.77 6,739 101,200 444 26.9 2,198
24 1.548 40.07 17.80 1,493 3,464 21.33 6,572 97,600 432 482,000 26.5 2,151
25 1.535 39.31 17.51 1,449 3,375 20.91 6,413 94,100 422 26.0 2,111
26 1.522 38.57 17.24 1,406 3,290 20.50 6,259 90,600 409 25.6 2,060
27 1.509 37.87 16.98 1,366 3,208 20.11 6,112 87,300 398 25.2 2,016
28 1.496 37.18 16.72 1,327 3,130 19.74 5,975 84,200 387 440,000 24.8 1,973
29 1.484 36.51 16.47 1,292 3,055 19.38 5,847 81,000 377 24.4 1,931
30 1.474 35.88 16.24 1,257 2,983 19.04 5,724 78,000 369 410,000 24.0 1,896
35 1.425 33.15 15.01 1,105 2,648 17.33 5,104 64,700 324 22.3 1,704
40 1.384 30.82 13.91 973 2,361 15.86 4,590 54,100 284 316,000 20.8 1,526
45 1.341 28.58 13.00 860 2,110 14.66 4,228 253 19.3 1,381
50 1.287 26.57 12.16 761 1,883 13.59 3,925 230 235,000 18.0 1,271
60 1.178 22.74 10.52 576 1,480 11.44 3,295 178 168,000 15.2 1,010
70 1.020 18.56 8.51 1,101 9.26 2,793 111,000 12.8
80 0.79 13.81 6.60 765 6.95 2,227 65,000 9.8
90 0.46 7.9 3.6 410 4.0 1,270 30,000 5.7
100 0 0 0 0 0 0 0 0
S
Notes: (1) The solubility form of Henry’s constant may be calculated as: H (mg i=LH 2 O)=atm i) ¼ Ae B (T C) (H.60) taking A and B from the table for the
i
gas of interest.
(2) All columns except O 3 and Rn were from Dean, J. A. (Ed.), Lange’s Handbook of Chemistry, 13th edn., McGraw-Hill, New York, 1985.
(continued)