Page 908 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 908
Appendix H: Dissolved Gases 863
10 6
NH 3
SO 2
10 5 4
Solubility of gases (mg/L) 10 3 CL 2 Rn S
H 2
10
CO 2
O
2
10
O 3 2
N 2
10 1
CH 4
H 2 CO
10 0
0 10 20 30 40 50 60 70
T (°C)
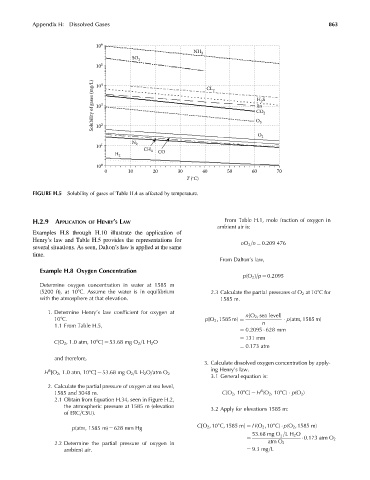
FIGURE H.5 Solubility of gases of Table H.4 as affected by temperature.
H.2.9 APPLICATION OF HENRY’S LAW From Table H.1, mole fraction of oxygen in
ambient air is:
Examples H.8 through H.10 illustrate the application of
Henry’s law and Table H.5 provides the representations for
nO 2 =n ¼ 0.209 476
several situations. As seen, Dalton’s law is applied at the same
time.
From Dalton’s law,
Example H.8 Oxygen Concentration
p(O 2 }=p ¼ 0.2095
Determine oxygen concentration in water at 1585 m
(5200 ft), at 108C. Assume the water is in equilibrium 2.3 Calculate the partial pressures of O 2 at 108C for
with the atmosphere at that elevation. 1585 m.
1. Determine Henry’s law coefficient for oxygen at n[O 2 , sea level]
108C. p[O 2 , 1585 m] ¼ p[atm, 1585 m]
1.1 From Table H.5, n
¼ 0:2095 628 mm
¼ 131 mm
C[O 2 , 1.0 atm, 108C] ¼ 53.68 mg O 2 =LH 2 O
¼ 0:173 atm
and therefore,
3. Calculate dissolved oxygen concentration by apply-
S ing Henry’s law.
H [O 2 , 1.0 atm, 108C] ¼ 53.68 mg O 2 =LH 2 O=atm O 2
3.1 General equation is:
2. Calculate the partial pressure of oxygen at sea level,
S
1585 and 3048 m. C[O 2 ,108C] ¼ H (O 2 ,108C) p(O 2 )
2.1 Obtain from Equation H.34, seen in Figure H.2,
the atmospheric pressure at 1585 m (elevation
3.2 Apply for elevations 1585 m:
of ERC=CSU).
C[O 2 ,10 C,1585 m] ¼ H(O 2 ,10 C) p(O 2 ,1585 m)
p(atm, 1585 m) ¼ 628 mm Hg
53:68 mg O =LH 2 O
2
0:173 atm O 2
¼
2.2 Determine the partial pressure of oxygen in atm O 2
ambient air. ¼ 9:3mg=L