Page 176 - Gas Purification 5E
P. 176
dlkanolamines fir Hydrogen Surjide and Carbon Dioxide Removal 163
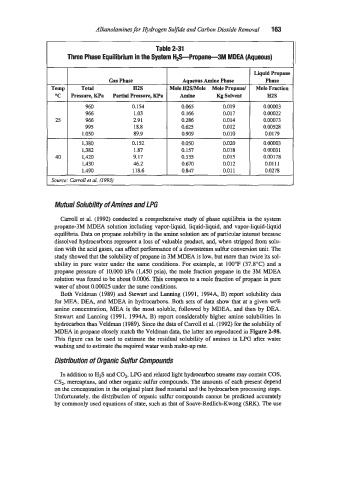
Table 2-31
Three Phase Equilibrium in the System H+Propane-3M MDEA (Aqueous)
Liquid Propane
Gas Phase Aqueous Amine Phase Phase
Temp Total H2S Mole H2SIMole Mole Propand Mole Fraction
OC Pressure, Wa Partial Pressure, KPa Amine Kg Solvent H2S
960 0.154 0.065 0.019 0.00003
966 1.03 0.166 0.017 0.00022
25 966 2.91 0.286 0.014 0.00073
995 18.8 0.625 0.012 0.00518
1,050 89.9 0.909 0.010 0.0179
1,380 0.152 0.050 0.020 0.00003
1,382 1.87 0.157 0.018 0.00031
40 1,420 9.17 0.335 0.015 0.00178
1,430 46.2 0.670 0.012 0.01 11
1,490 118.6 0.847 0.01 1 0.0278
Source: Carroll et al. (1 993)
Mutual Solubility of Amines and LPG
Carroll et al. (1992) conducted a comprehensive study of phase equilibria in the system
propane-3M MDEA solution including vapor-liquidl liquid-liquid, and vapor-liquid-liquid
equilibria. Data on propane solubility in the amine solution are of particular interest because
dissolved hydrocarbons represent a loss of valuable product, and, when stripped from solu-
tion with the acid gases, can affect performance of a downstream sulfur conversion unit. The
study showed that the solubility of propane in 3M MDEA is low, but more than twice its sol-
ubility in pure water under the same conditions. For example, at 100°F (37.8"C) and a
propane pressure of 10,000 Ea (1,450 psia), the mole fraction propane in the 3M MDEA
solution was found to be about 0.0006. This compares to a mole fraction of propane in pure
water of about 0.00025 under the same conditions.
Both Veldman (1989) and Stewart and Lanning (1991, 1994A, B) report solubility data
for MEA, DEA, and MDEA in hydrocarbons. Both sets of data show that at a given wt%,
amine concentration, MEA is the most soluble, followed by MDEA, and then by DEA.
Stewart and Lanning (1991, 1994A, B) report considerably higher amine solubilities in
hydrocarbon than Veldman (1989). Since the data of Carroll et al. (1992) for the solubility of
MDEA in propane closely match the Veldman data, the latter are reproduced in Figure 2-98.
This figure can be used to estimate the residual solubility of amines in LPG after water
washing and to estimate the required water wash make-up rate.
Distribution of Organic Sulfur Compounds
In addition to H2S and COz, LPG and related light hydrocarbon streams may contain COS,
CS2, mercaptans, and other organic sulfur compounds. The amounts of each present depend
on the concentration in the original plant feed material and the hydrocarbon processing steps.
Unfortunately, thz distribution of organic sulfur compounds cannot be predicted accurately
by commonly used equations of state, such as that of Soave-Redlich-Kwong (SRK). The use