Page 177 - Gas Purification 5E
P. 177
164 Gas PziriJication
100
90
80
:m
L
U
660
I
:=
T
y40
P
P
M30
W
20
10
0
WT% ETHANOLAMINE IN AQUEOUS SOLUTION
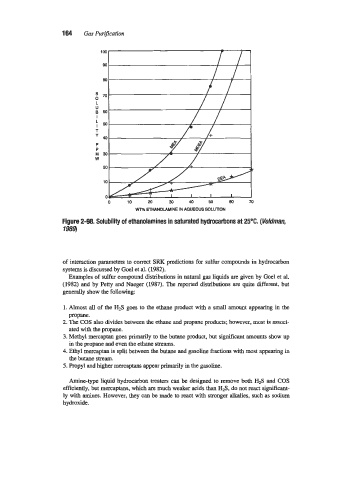
Figure 2-98. Solubility of ethanolamines in saturated hydrocahons at 25°C. (Ve/dman,
1989
of interaction parameters to correct SRK predictions for sulfur compounds in hydrocarbon
systems is discussed by Goel et al. (1982).
Examples of sulfur compound distributions in natural gas liquids are given by Goel et al.
(1982) and by Petty and Naeger (1987). The reported distributions are quite different, but
generally show the following:
1. Almost all of the H2S goes to the ethane product with a small amount appearing in the
propane.
2. The COS also divides between the ethane and propane products; however, most is associ-
ated with the propane.
3. Methyl mercaptan goes primarily to the butane product, but significant amounts show up
in the propane and even the ethane streams.
4. Ethyl mercaptan is split between the butane and gasoline fractions with most appearing in
the butane stream.
5. Propyl and higher mercaptans appear primarily in the gasoline.
Amine-type liquid hydrocarbon treaters can be designed to remove both H2S and COS
efficiently, but mercaptans, which are much weaker acids than H2S, do not react significant-
ly with amines. However, they can be made to react with stronger alkalies, such as sodium
hydroxide.