Page 222 - Gas Purification 5E
P. 222
208 Gas Purification
Boy:
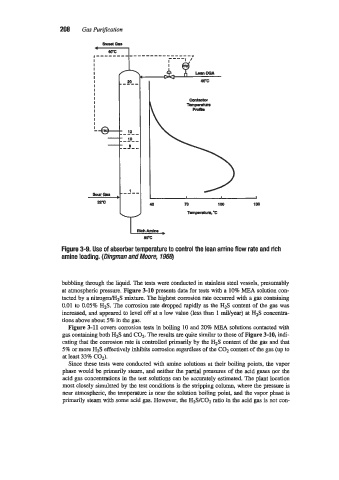
Figure 3-9. Use of absorber temperature to conirol the lean amine flow rate and rich
amine loading. (Dingman and Moore, 1968)
bubbling through the liquid. The tests were conducted in stainless steel vessels, presumably
at atmospheric pressure. Figure 3-10 presents data for tests with a 10% MEA solution con-
tacted by a nitmgen/H2S mixture. The highest corrosion rate occurred with a gas containing
0.01 to 0.05% H2S. The corrosion rate dropped rapidly as the H2S content of the gas was
increased, and appeared to level off at a low value (less than 1 dyear) at H2S concentra-
tions above about 5% in the gas.
Figure 3-11 covers corrosion tests in boiling 10 and 20% MEA solutions contacted with
gas containing both H2S and C02. The results are quite similar to those of Figure 3-10, indi-
cating that the corrosion rate is controlled primarily by the H2S content of the gas and that
5% or more H2S effectively inhibits corrosion regardless of the C02 content of the gas (up to
at least 33% C02).
Since these tests were conducted with amine solutions at their boiling points, the vapor
phase would be primarily steam, and neither the partial pressures of the acid gases nor the
acid gas concentrations in the test solutions can be accurately estimated. The plant location
most closely simulated by the test conditions is the stripping column, where the pressure is
near atmospheric, the temperature is near the solution boiling point, and the vapor phase is
primarily steam with some acid gas. However, the H2S/C02 ratio in the acid gas is not con-