Page 224 - Gas Purification 5E
P. 224
21 0 Gas Purifxation
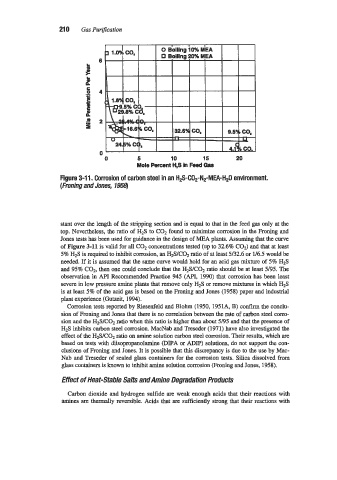
0 5 10 15 20
Mole Percent H,S in Feed Gas
Figure 3-1 1. Corrosion of carbon steel in an H2S-CO2-N2-MEA-H2O environment.
(Froning and Jones, 7958)
stant over the length of the stripping section and is equal to that in the feed gas only at the
top. Nevertheless, the ratio of H2S to COz found to minimize corrosion in the Froning and
Jones tests has been used for guidance in the design of MEA plants. Assuming that the curve
of Figure 3-11 is valid for all C02 concentrations tested (up to 32.6% COz) and that at least
5% H2S is required to inhibit corrosion, an H2S/C02 ratio of at least Y32.6 or U6.5 would be
needed. If it is assumed that the same curve would hold for an acid gas mixture of 5% H2S
and 95% COz, then one could conclude that the HzSIC02 ratio should be at least 5/95. The
observation in API Recommended Practice 945 (API, 1990) that corrosion has been least
severe in low pressure amine plants that remove only H2S or remove mixtures in which H2S
is at least 5% of the acid gas is based on the Froning and Jones (1958) paper and industrial
plant experience (Gutzeit, 1994).
Corrosion tests reported by Riesenfeld and Blohm (1950, 1951A, B) confirm the conclu-
sion of Froning and Jones that there is no correlation between the rate of carbon steel corro-
sion and the H2S/C02 ratio when this ratio is higher than about 5/95 and that the presence of
H2S inhibits carbon steel corrosion. MacNab and Treseder (197 1) have also investigated the
effect of the H2S/C02 ratio on amine solution carbon steel corrosion. Their results, which are
based on tests with diisopropanolamhe @PA or ADP) solutions, do not support the con-
clusions of Froning and Jones. It is possible that this discrepancy is due to the use by Mac-
Nab and Treseder of sealed glass containers for the corrosion tests. Silica dissolved from
glass containers is known to inhibit amine solution corrosion (Froning and Jones, 1958).
Effect of Heat-Stable Salts and Amine Degradation Products
Carbon dioxide and hydrogen sulfide are weak enough acids that their reactions with
amines are thermally reversible. Acids that are sufficiently strong that their reactions Virith