Page 283 - Gas Purification 5E
P. 283
Mechanical Design and Operation of Alkanolarnine Plants 267
regeneration section of the plant. This is particularly undesirable if the acid gases are intend-
ed to be used further, as for instance, for the production of dry ice or elemental sulfur, The
nonacidic gas may be carried both in solution and as entrained bubbles (or as drops of liquid
hydrocarbon). Mechanical canyover of nonacidic gases can be minimized by proper design
of the bottom section of the contactor. Both splashing and the free fall of liquids through the
vapor space (which result in hth in the bottom of the contactor) should be avoided by the
installation of a properly designed downcomer, and the outlet line should include a vortex
breaker. Even when the contactor bottom is properly designed, it is in most cases impossible
to eliminate totally mechanical canyover. In addition, the solubility of most nonacidic gases
in ethanolamine solutions becomes appreciable at high pressures. Provisions, therefore, must
be made to separate these gases from the solution after it leaves the contactor and before it
enters the regenerating section. Depending on the operating conditions of the plant and the
purity requirements of the acid gas, a rich amine flash drum is often installed downstream of
the rich solution level control valve and upstream of the lean-to-rich solution heat exchanger.
To provide a maximum of vapor-disengaging area, horizontal vessels are frequently used.
The gases evolved in the rich amine flash drum contain acid gas in varying concentrations.
This acid gas can be recovered by contacting the flashed gases with a small stream of lean
amine solution in a small column installed at the top of the flash drum (see Chapter 2).
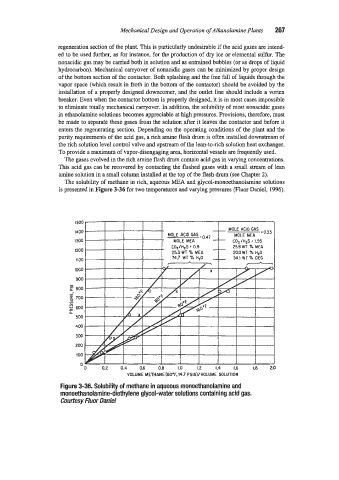
The solubility of methane in rich, aqueous MEA and glycol-monoethanolamine solutions
is presented in Figure 3-36 for two temperatures and varying pressures (Fluor Daniel, 1996).
VOLUME METHANE (60*F, 14.7 PSIAIIVOLUME SOLUTION
Ftgure 3-36. Solubility of methane in aqueous monoethanolamine and
monoethanolamine-diethylene glycol-water solutions containing acid gas.
Courtesy Fluor Daniel