Page 148 - Gas Wettability of Reservoir Rock Surfaces with Porous Media
P. 148
132 Gas Wettability of Reservoir Rock Surfaces with Porous Media
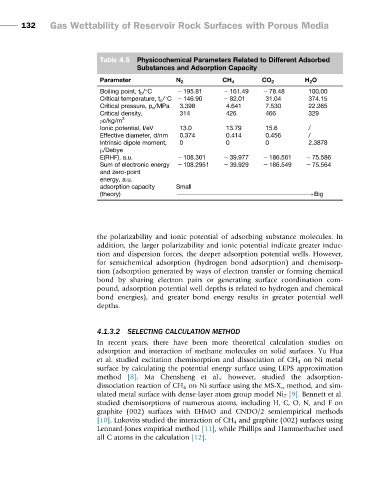
Table 4.5 Physicochemical Parameters Related to Different Adsorbed
Substances and Adsorption Capacity
Parameter N 2 CH 4 CO 2 H 2 O
Boiling point, t b / C 2 195.81 2 161.49 2 78.48 100.00
Critical temperature, t c / C 2 146.90 2 82.01 31.04 374.15
Critical pressure, p c /MPa 3.398 4.641 7.530 22.265
Critical density, 314 426 466 329
ρc/kg/m 3
Ionic potential, I/eV 13.0 13.79 15.6 /
Effective diameter, d/nm 0.374 0.414 0.456 /
Intrinsic dipole moment, 0 0 0 2.3878
μ/Debye
E(RHF), a.u. 2 108.301 2 39.977 2 186.561 2 75.586
Sum of electronic energy 2 108.2951 2 39.929 2 186.549 2 75.564
and zero-point
energy, a.u.
adsorption capacity Small
(theory) !Big
the polarizability and ionic potential of adsorbing substance molecules. In
addition, the larger polarizability and ionic potential indicate greater induc-
tion and dispersion forces, the deeper adsorption potential wells. However,
for semichemical adsorption (hydrogen bond adsorption) and chemisorp-
tion (adsorption generated by ways of electron transfer or forming chemical
bond by sharing electron pairs or generating surface coordination com-
pound, adsorption potential well depths is related to hydrogen and chemical
bond energies), and greater bond energy results in greater potential well
depths.
4.1.3.2 SELECTING CALCULATION METHOD
In recent years, there have been more theoretical calculation studies on
adsorption and interaction of methane molecules on solid surfaces. Yu Hua
et al. studied excitation chemisorption and dissociation of CH 4 on Ni metal
surface by calculating the potential energy surface using LEPS approximation
method [8]. Ma Chensheng et al., however, studied the adsorption-
dissociation reaction of CH 4 on Ni surface using the MS-X α method, and sim-
ulated metal surface with dense-layer atom group model Ni 7 [9]. Bennett et al.
studied chemisorptions of numerous atoms, including H, C, O, N, and F on
graphite (002) surfaces with EHMO and CNDO/2 semiempirical methods
[10]. Lukovits studied the interaction of CH 4 and graphite (002) surfaces using
Lennard-Jones empirical method [11], while Phillips and Hammerbacher used
all C atoms in the calculation [12].