Page 150 - Gas Wettability of Reservoir Rock Surfaces with Porous Media
P. 150
134 Gas Wettability of Reservoir Rock Surfaces with Porous Media
4.1.3.3.2 Models of Gas-wetting Rocks
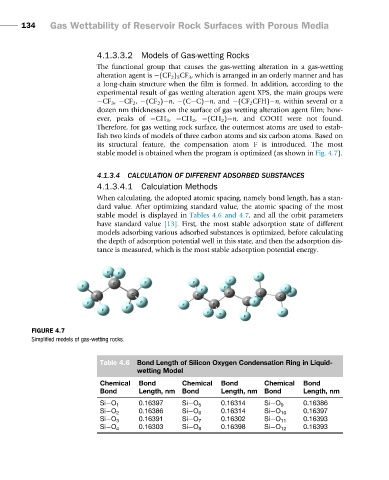
The functional group that causes the gas-wetting alteration in a gas-wetting
alteration agent is (CF 2 ) 5 CF 3 , which is arranged in an orderly manner and has
a long-chain structure when the film is formed. In addition, according to the
experimental result of gas wetting alteration agent XPS, the main groups were
CF 3 , CF 2 , (CF 2 ) n, (C C) n, and (CF 2 CFH) n, within several or a
dozen nm thicknesses on the surface of gas wetting alteration agent film; how-
ever, peaks of CH 3 , CH 2 , (CH 2 ) n, and COOH were not found.
Therefore, for gas wetting rock surface, the outermost atoms are used to estab-
lish two kinds of models of three carbon atoms and six carbon atoms. Based on
its structural feature, the compensation atom F is introduced. The most
stable model is obtained when the program is optimized (as shown in Fig. 4.7).
4.1.3.4 CALCULATION OF DIFFERENT ADSORBED SUBSTANCES
4.1.3.4.1 Calculation Methods
When calculating, the adopted atomic spacing, namely bond length, has a stan-
dard value. After optimizing standard value, the atomic spacing of the most
stable model is displayed in Tables 4.6 and 4.7, and all the orbit parameters
have standard value [13]. First, the most stable adsorption state of different
models adsorbing various adsorbed substances is optimized, before calculating
the depth of adsorption potential well in this state, and then the adsorption dis-
tance is measured, which is the most stable adsorption potential energy.
FIGURE 4.7
Simplified models of gas-wetting rocks.
Table 4.6 Bond Length of Silicon Oxygen Condensation Ring in Liquid-
wetting Model
Chemical Bond Chemical Bond Chemical Bond
Bond Length, nm Bond Length, nm Bond Length, nm
Si O 1 0.16397 Si O 5 0.16314 Si O 9 0.16386
Si O 2 0.16386 Si O 6 0.16314 Si O 10 0.16397
Si O 3 0.16391 Si O 7 0.16302 Si O 11 0.16393
0.16303 0.16398 0.16393
Si O 4 Si O 8 Si O 12