Page 156 - Gas Wettability of Reservoir Rock Surfaces with Porous Media
P. 156
140 Gas Wettability of Reservoir Rock Surfaces with Porous Media
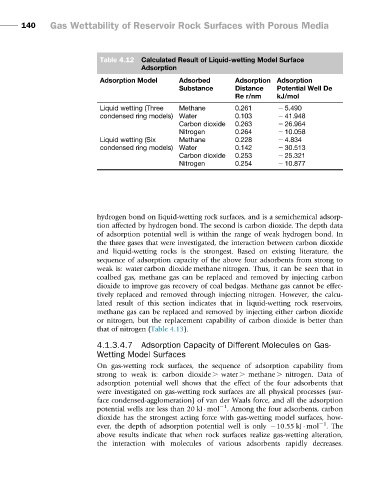
Table 4.12 Calculated Result of Liquid-wetting Model Surface
Adsorption
Adsorption Model Adsorbed Adsorption Adsorption
Substance Distance Potential Well De
Re r/nm kJ/mol
Liquid wetting (Three Methane 0.261 2 5.490
condensed ring models) Water 0.103 2 41.948
Carbon dioxide 0.263 2 26.964
Nitrogen 0.264 2 10.058
Liquid wetting (Six Methane 0.228 2 4.834
condensed ring models) Water 0.142 2 30.513
Carbon dioxide 0.253 2 25.321
Nitrogen 0.254 2 10.877
hydrogen bond on liquid-wetting rock surfaces, and is a semichemical adsorp-
tion affected by hydrogen bond. The second is carbon dioxide. The depth data
of adsorption potential well is within the range of weak hydrogen bond. In
the three gases that were investigated, the interaction between carbon dioxide
and liquid-wetting rocks is the strongest. Based on existing literature, the
sequence of adsorption capacity of the above four adsorbents from strong to
weak is: water>carbon dioxide>methane>nitrogen. Thus, it can be seen that in
coalbed gas, methane gas can be replaced and removed by injecting carbon
dioxide to improve gas recovery of coal bedgas. Methane gas cannot be effec-
tively replaced and removed through injecting nitrogen. However, the calcu-
lated result of this section indicates that in liquid-wetting rock reservoirs,
methane gas can be replaced and removed by injecting either carbon dioxide
or nitrogen, but the replacement capability of carbon dioxide is better than
that of nitrogen (Table 4.13).
4.1.3.4.7 Adsorption Capacity of Different Molecules on Gas-
Wetting Model Surfaces
On gas-wetting rock surfaces, the sequence of adsorption capability from
strong to weak is: carbon dioxide . >water . >methane . >nitrogen. Data of
adsorption potential well shows that the effect of the four adsorbents that
were investigated on gas-wetting rock surfaces are all physical processes (sur-
face condensed-agglomeration) of van der Waals force, and all the adsorption
potential wells are less than 20 kJ mol 21 . Among the four adsorbents, carbon
dioxide has the strongest acting force with gas-wetting model surfaces, how-
ever, the depth of adsorption potential well is only 210.55 kJ mol 21 . The
above results indicate that when rock surfaces realize gas-wetting alteration,
the interaction with molecules of various adsorbents rapidly decreases.