Page 159 - Gas Wettability of Reservoir Rock Surfaces with Porous Media
P. 159
Effect of Gas Wettability on the Surface Properties CHAPTER 4 143
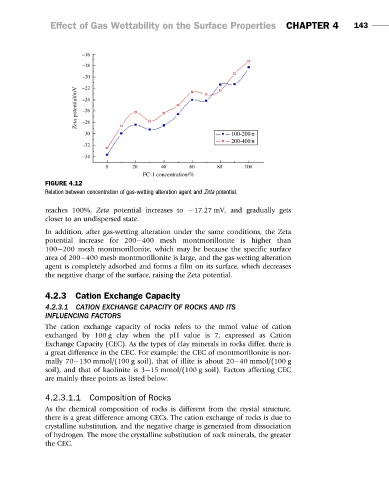
FIGURE 4.12
Relation between concentration of gas-wetting alteration agent and Zeta potential.
reaches 100%, Zeta potential increases to 217.27 mV, and gradually gets
closer to an undispersed state.
In addition, after gas-wetting alteration under the same conditions, the Zeta
potential increase for 200 400 mesh montmorillonite is higher than
100 200 mesh montmorillonite, which may be because the specific surface
area of 200 400 mesh montmorillonite is large, and the gas-wetting alteration
agent is completely adsorbed and forms a film on its surface, which decreases
the negative charge of the surface, raising the Zeta potential.
4.2.3 Cation Exchange Capacity
4.2.3.1 CATION EXCHANGE CAPACITY OF ROCKS AND ITS
INFLUENCING FACTORS
The cation exchange capacity of rocks refers to the mmol value of cation
exchanged by 100 g clay when the pH value is 7, expressed as Cation
Exchange Capacity (CEC). As the types of clay minerals in rocks differ, there is
a great difference in the CEC. For example: the CEC of montmorillonite is nor-
mally 70 130 mmol/(100 g soil), that of illite is about 20 40 mmol/(100 g
soil), and that of kaolinite is 3 15 mmol/(100 g soil). Factors affecting CEC
are mainly three points as listed below:
4.2.3.1.1 Composition of Rocks
As the chemical composition of rocks is different from the crystal structure,
there is a great difference among CECs. The cation exchange of rocks is due to
crystalline substitution, and the negative charge is generated from dissociation
of hydrogen. The more the crystalline substitution of rock minerals, the greater
the CEC.