Page 59 - Gas Wettability of Reservoir Rock Surfaces with Porous Media
P. 59
Evaluation Methods and Influencing Factors CHAPTER 2 43
30
20
Water
10 Oil
0
h/mm –10
–20
–30
–40
–50
–60
0 1000 2000 3000 4000 5000
–1
FG40 concentration/(mg*L )
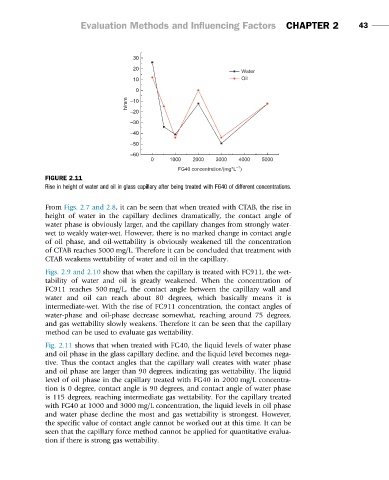
FIGURE 2.11
Rise in height of water and oil in glass capillary after being treated with FG40 of different concentrations.
From Figs. 2.7 and 2.8, it can be seen that when treated with CTAB, the rise in
height of water in the capillary declines dramatically, the contact angle of
water phase is obviously larger, and the capillary changes from strongly water-
wet to weakly water-wet. However, there is no marked change in contact angle
of oil phase, and oil-wettability is obviously weakened till the concentration
of CTAB reaches 5000 mg/L. Therefore it can be concluded that treatment with
CTAB weakens wettability of water and oil in the capillary.
Figs. 2.9 and 2.10 show that when the capillary is treated with FC911, the wet-
tability of water and oil is greatly weakened. When the concentration of
FC911 reaches 500 mg/L, the contact angle between the capillary wall and
water and oil can reach about 80 degrees, which basically means it is
intermediate-wet. With the rise of FC911 concentration, the contact angles of
water-phase and oil-phase decrease somewhat, reaching around 75 degrees,
and gas wettability slowly weakens. Therefore it can be seen that the capillary
method can be used to evaluate gas wettability.
Fig. 2.11 shows that when treated with FG40, the liquid levels of water phase
and oil phase in the glass capillary decline, and the liquid level becomes nega-
tive. Thus the contact angles that the capillary wall creates with water phase
and oil phase are larger than 90 degrees, indicating gas wettability. The liquid
level of oil phase in the capillary treated with FG40 in 2000 mg/L concentra-
tion is 0 degree, contact angle is 90 degrees, and contact angle of water phase
is 115 degrees, reaching intermediate gas wettability. For the capillary treated
with FG40 at 1000 and 3000 mg/L concentration, the liquid levels in oil phase
and water phase decline the most and gas wettability is strongest. However,
the specific value of contact angle cannot be worked out at this time. It can be
seen that the capillary force method cannot be applied for quantitative evalua-
tion if there is strong gas wettability.