Page 57 - Gas Wettability of Reservoir Rock Surfaces with Porous Media
P. 57
Evaluation Methods and Influencing Factors CHAPTER 2 41
2.2.1 Evaluating Gas Wettability by Capillary Force Method
2.2.1.1 EXPERIMENTAL METHOD
The capillary is soaked in chromic acid solution for 24 hours, then washed
with distilled water. CTAB, FC911, and FG40 solutions are prepared separately
with 500, 1000, 2000, 3000, 4000, and 5000 mg/L concentrations. Chemical
solutions are imbibed into the capillary, then dried after two hours. The
capillaries are inserted in 0.2% NaCl solution and decane, respectively.
The rise in height of NaCl solution and decane is observed after they stabilize.
The equation of contact angle is as follows:
ρghr
θ 5 arccosθ (2.12)
2σ
In the equation, θ 5 contact angle in degrees.
3
ρ is density of liquid, in kg/m .
2
g is acceleration of gravity, taken as 9.8 m/s .
h is rising height of liquid in capillary in cm.
r is capillary radius in m.
σ is surface tension of liquid in N/m.
2.2.1.2 EXPERIMENTAL RESULT AND DISCUSSION
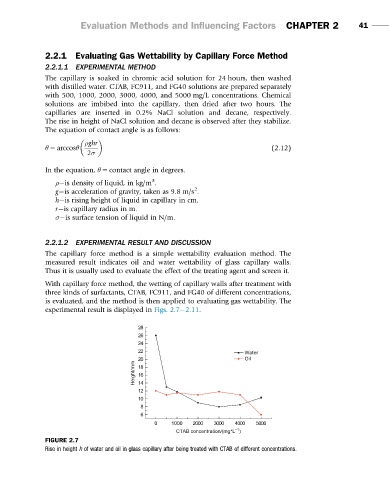
The capillary force method is a simple wettability evaluation method. The
measured result indicates oil and water wettability of glass capillary walls.
Thus it is usually used to evaluate the effect of the treating agent and screen it.
With capillary force method, the wetting of capillary walls after treatment with
three kinds of surfactants, CTAB, FC911, and FG40 of different concentrations,
is evaluated, and the method is then applied to evaluating gas wettability. The
experimental result is displayed in Figs. 2.7 2.11.
28
26
24
22 Water
20 Oil
Height/mm 18
16
14
12
10
8
6
0 1000 2000 3000 4000 5000
–1
CTAB concentration/(mg*L )
FIGURE 2.7
Rise in height h of water and oil in glass capillary after being treated with CTAB of different concentrations.