Page 53 - Gas Wettability of Reservoir Rock Surfaces with Porous Media
P. 53
Evaluation Methods and Influencing Factors CHAPTER 2 37
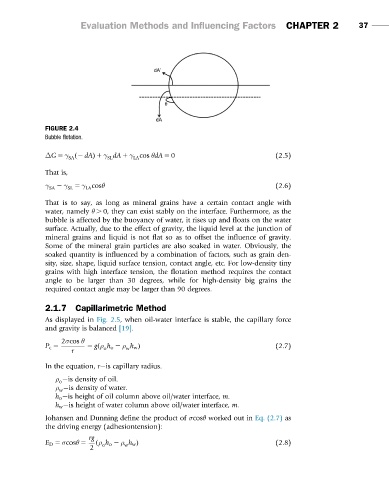
FIGURE 2.4
Bubble flotation.
ΔG 5 γ ð2 dAÞ 1 γ dA 1 γ cos θdA 5 0 (2.5)
SA SL LA
That is,
γ 2 γ 5 γ cosθ (2.6)
SA SL LA
That is to say, as long as mineral grains have a certain contact angle with
water, namely θ . 0, they can exist stably on the interface. Furthermore, as the
bubble is affected by the buoyancy of water, it rises up and floats on the water
surface. Actually, due to the effect of gravity, the liquid level at the junction of
mineral grains and liquid is not flat so as to offset the influence of gravity.
Some of the mineral grain particles are also soaked in water. Obviously, the
soaked quantity is influenced by a combination of factors, such as grain den-
sity, size, shape, liquid surface tension, contact angle, etc. For low-density tiny
grains with high interface tension, the flotation method requires the contact
angle to be larger than 30 degrees, while for high-density big grains the
required contact angle may be larger than 90 degrees.
2.1.7 Capillarimetric Method
As displayed in Fig. 2.5, when oil-water interface is stable, the capillary force
and gravity is balanced [19].
2σcos θ
P c 5 5 gðρ h o 2 ρ h w Þ (2.7)
w
o
r
In the equation, r is capillary radius.
ρ is density of oil.
o
ρ is density of water.
w
h o is height of oil column above oil/water interface, m.
h w is height of water column above oil/water interface, m.
Johansen and Dunning define the product of σcosθ worked out in Eq. (2.7) as
the driving energy (adhesiontension):
rg
E D 5 σcosθ 5 ðρ h o 2 ρ h w Þ (2.8)
w
o
2