Page 211 - Gas Adsorption Equilibria
P. 211
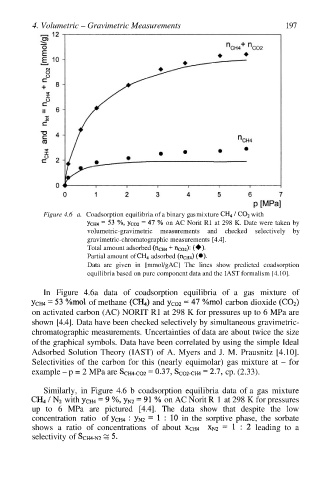
4. Volumetric – Gravimetric Measurements 197
Figure 4.6 a. Coadsorption equilibria of a binary gas mixture with
on AC Norit R1 at 298 K. Date were taken by
volumetric-gravimetric measurements and checked selectively by
gravimetric-chromatographic measurements [4.4].
Total amount adsorbed
Partial amount of adsorbed
Data are given in [mmol/gAC] The lines show predicted coadsorption
equilibria based on pure component data and the IAST formalism [4.10].
In Figure 4.6a data of coadsorption equilibria of a gas mixture of
of methane and carbon dioxide
on activated carbon (AC) NORIT R1 at 298 K for pressures up to 6 MPa are
shown [4.4]. Data have been checked selectively by simultaneous gravimetric-
chromatographic measurements. Uncertainties of data are about twice the size
of the graphical symbols. Data have been correlated by using the simple Ideal
Adsorbed Solution Theory (IAST) of A. Myers and J. M. Prausnitz [4.10].
Selectivities of the carbon for this (nearly equimolar) gas mixture at – for
example – p = 2 MPa are cp. (2.33).
Similarly, in Figure 4.6 b coadsorption equilibria data of a gas mixture
with on AC Norit R 1 at 298 K for pressures
up to 6 MPa are pictured [4.4]. The data show that despite the low
concentration ratio of in the sorptive phase, the sorbate
shows a ratio of concentrations of about leading to a
selectivity of