Page 213 - Gas Adsorption Equilibria
P. 213
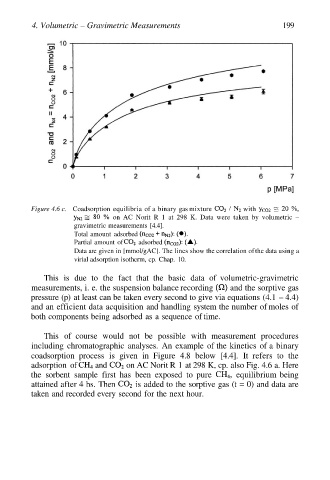
4. Volumetric – Gravimetric Measurements 199
Figure 4.6 c. Coadsorption equilibria of a binary gas mixture with
on AC Norit R 1 at 298 K. Data were taken by volumetric –
gravimetric measurements [4.4].
Total amount adsorbed
Partial amount of adsorbed
Data are given in [mmol/gAC]. The lines show the correlation of the data using a
virial adsorption isotherm, cp. Chap. 10.
This is due to the fact that the basic data of volumetric-gravimetric
measurements, i. e. the suspension balance recording and the sorptive gas
pressure (p) at least can be taken every second to give via equations (4.1 – 4.4)
and an efficient data acquisition and handling system the number of moles of
both components being adsorbed as a sequence of time.
This of course would not be possible with measurement procedures
including chromatographic analyses. An example of the kinetics of a binary
coadsorption process is given in Figure 4.8 below [4.4]. It refers to the
adsorption of and on AC Norit R 1 at 298 K, cp. also Fig. 4.6 a. Here
the sorbent sample first has been exposed to pure equilibrium being
attained after 4 hs. Then is added to the sorptive gas (t = 0) and data are
taken and recorded every second for the next hour.