Page 98 - Geochemical Remote Sensing of The Sub-Surface
P. 98
Geoelectrochemistry and stream dispersion 75
1
o~, i
~,v
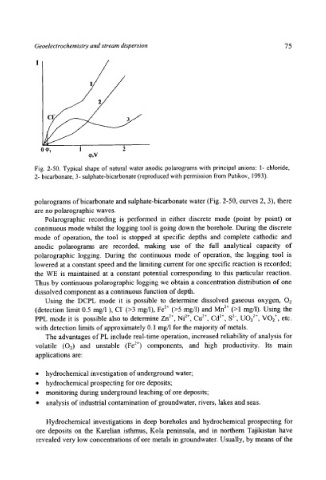
Fig. 2-50. Typical shape of natural water anodic polarograms with principal anions: 1- chloride,
2- bicarbonate, 3- sulphate-bicarbonate (reproduced with permission from Putikov, 1993).
polarograms of bicarbonate and sulphate-bicarbonate water (Fig. 2-50, curves 2, 3), there
are no polarographic waves.
Polarographic recording is performed in either discrete mode (point by point) or
continuous mode whilst the logging tool is going down the borehole. During the discrete
mode of operation, the tool is stopped at specific depths and complete cathodic and
anodic polarograms are recorded, making use of the full analytical capacity of
polarographic logging. During the continuous mode of operation, the logging tool is
lowered at a constant speed and the limiting current for one specific reaction is recorded;
the WE is maintained at a constant potential corresponding to this particular reaction.
Thus by continuous polarographic logging we obtain a concentration distribution of one
dissolved component as a continuous function of depth.
Using the DCPL mode it is possible to determine dissolved gaseous oxygen, 02
(detection limit 0.5 mg/1 ), C1- (>3 mg/l), Fe 2+ (>5 mg/1) and Mn 2§ (>1 mg/1). Using the
PPL mode it is possible also to determine Zn 2§ Ni 2§ Cu 2+, Cd 2+, S 2-, UO2 2+, VO2 +, etc.
with detection limits of approximately 0.1 mg/l for the majority of metals.
The advantages of PL include real-time operation, increased reliability of analysis for
volatile (02) and unstable (Fe 2+) components, and high productivity. Its main
applications are:
9 hydrochemical investigation of underground water;
9 hydrochemical prospecting for ore deposits;
9 monitoring during underground leaching of ore deposits;
9 analysis of industrial contamination of groundwater, rivers, lakes and seas.
Hydrochemical investigations in deep boreholes and hydrochemical prospecting for
ore deposits on the Karelian isthmus, Kola peninsula, and in northern Tajikistan have
revealed very low concentrations of ore metals in groundwater. Usually, by means of the