Page 104 - Geothermal Energy Renewable Energy and The Environment
P. 104
Chemistry of Geothermal Fluids 89
–10.0
–11.0
5.81 5.58
5.64 5.65
Log K –12.0 6.13
–13.0 6.50
–14.0
0 50 100 150 200 250 300 350
Temperature (°C )
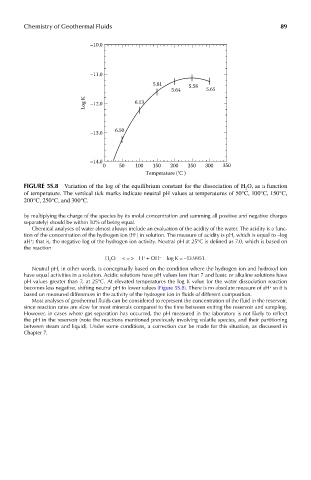
FIGUre 5s.8 Variation of the log of the equilibrium constant for the dissociation of H 2 O, as a function
of temperature. The vertical tick marks indicate neutral pH values at temperatures of 50°C, 100°C, 150°C,
200°C, 250°C, and 300°C.
by multiplying the charge of the species by its molal concentration and summing all positive and negative charges
separately) should be within 10% of being equal.
Chemical analyses of water almost always include an evaluation of the acidity of the water. The acidity is a func-
+
tion of the concentration of the hydrogen ion (H ) in solution. The measure of acidity is pH, which is equal to –log
aH ; that is, the negative log of the hydrogen ion activity. Neutral pH at 25°C is defined as 7.0, which is based on
+
the reaction
+
–
H 2 O < = > H + OH log K = –13.9951.
Neutral pH, in other words, is conceptually based on the condition where the hydrogen ion and hydroxyl ion
have equal activities in a solution. Acidic solutions have pH values less than 7 and basic or alkaline solutions have
pH values greater than 7, at 25°C. At elevated temperatures the log K value for the water dissociation reaction
becomes less negative, shifting neutral pH to lower values (Figure 5S.8). There is no absolute measure of aH so it is
+
based on measured differences in the activity of the hydrogen ion in fluids of different composition.
Most analyses of geothermal fluids can be considered to represent the concentration of the fluid in the reservoir,
since reaction rates are slow for most minerals compared to the time between exiting the reservoir and sampling.
However, in cases where gas separation has occurred, the pH measured in the laboratory is not likely to reflect
the pH in the reservoir (note the reactions mentioned previously involving volatile species, and their partitioning
between steam and liquid). Under some conditions, a correction can be made for this situation, as discussed in
Chapter 7.