Page 216 - Geothermal Energy Renewable Energy and The Environment
P. 216
204 Geothermal Energy: Renewable Energy and the Environment
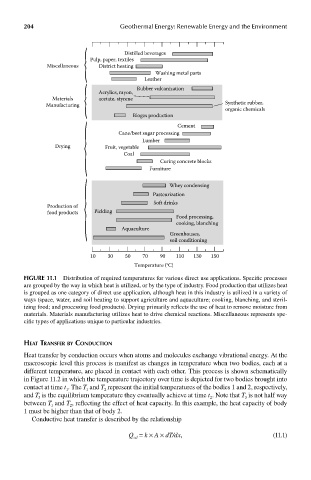
Distilled beverages
Pulp, paper, textiles
Miscellaneous District heating
Washing metal parts
Leather
Rubber vulcanization
Acrylics, rayon,
Materials acetate, styrene
Manufacturing Synthetic rubber,
organic chemicals
Biogas production
Cement
Cane/beet sugar processing
Lumber
Drying Fruit, vegetable
Coal
Curing concrete blocks
Furniture
Whey condensing
Pasteurization
Soft drinks
Production of
food products Pickling
Food processing,
cooking, blanching
Aquaculture
Greenhouses,
soil conditioning
10 30 50 70 90 110 130 150
Temperature (°C)
FIGUre 11.1 Distribution of required temperatures for various direct use applications. Specific processes
are grouped by the way in which heat is utilized, or by the type of industry. Food production that utilizes heat
is grouped as one category of direct use application, although heat in this industry is utilized in a variety of
ways (space, water, and soil heating to support agriculture and aquaculture; cooking, blanching, and steril-
izing food; and processing food products). Drying primarily reflects the use of heat to remove moisture from
materials. Materials manufacturing utilizes heat to drive chemical reactions. Miscellaneous represents spe-
cific types of applications unique to particular industries.
heaT Transfer by conducTion
Heat transfer by conduction occurs when atoms and molecules exchange vibrational energy. At the
macroscopic level this process is manifest as changes in temperature when two bodies, each at a
different temperature, are placed in contact with each other. This process is shown schematically
in Figure 11.2 in which the temperature trajectory over time is depicted for two bodies brought into
contact at time t . The T and T represent the initial temperatures of the bodies 1 and 2, respectively,
1
1
2
and T is the equilibrium temperature they eventually achieve at time t . Note that T is not half way
3
3
2
between T and T , reflecting the effect of heat capacity. In this example, the heat capacity of body
1
2
1 must be higher than that of body 2.
Conductive heat transfer is described by the relationship
Q = k × A × dT/dx, (11.1)
cd