Page 34 - Geothermal Energy Renewable Energy and The Environment
P. 34
Sources of Geothermal Heat: Earth as a Heat Engine 17
7.0
Quartz
6.0
Thermal conductivity (W/m–K) 4.0 Limestone Granite
5.0
3.0
2.0
Alkali feldspar
Dry sand Basalt
Water
1.0
Air
0.0
0 50 100 150 200 250
Temperature (°C)
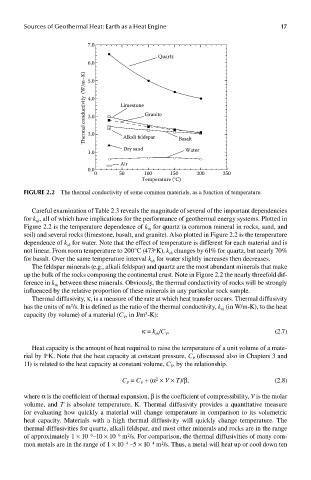
FIGUre 2.2 The thermal conductivity of some common materials, as a function of temperature.
Careful examination of Table 2.3 reveals the magnitude of several of the important dependencies
for k , all of which have implications for the performance of geothermal energy systems. Plotted in
th
Figure 2.2 is the temperature dependence of k for quartz (a common mineral in rocks, sand, and
th
soil) and several rocks (limestone, basalt, and granite). Also plotted in Figure 2.2 is the temperature
dependence of k for water. Note that the effect of temperature is different for each material and is
th
not linear. From room temperature to 200°C (473 K), k changes by 61% for quartz, but nearly 70%
o
th
for basalt. Over the same temperature interval k for water slightly increases then decreases.
th
The feldspar minerals (e.g., alkali feldspar) and quartz are the most abundant minerals that make
up the bulk of the rocks composing the continental crust. Note in Figure 2.2 the nearly threefold dif-
ference in k between these minerals. Obviously, the thermal conductivity of rocks will be strongly
th
influenced by the relative proportion of these minerals in any particular rock sample.
Thermal diffusivity, κ, is a measure of the rate at which heat transfer occurs. Thermal diffusivity
has the units of m /s. It is defined as the ratio of the thermal conductivity, k (in W/m-K), to the heat
2
th
capacity (by volume) of a material (C , in J/m -K):
3
V
κ = k /C . (2.7)
V
th
Heat capacity is the amount of heat required to raise the temperature of a unit volume of a mate-
rial by 1 K. Note that the heat capacity at constant pressure, C (discussed also in Chapters 3 and
o
P
11) is related to the heat capacity at constant volume, C , by the relationship.
V
C = C + (α × V × T)/β, (2.8)
2
P
V
where α is the coefficient of thermal expansion, β is the coefficient of compressibility, V is the molar
volume, and T is absolute temperature, K. Thermal diffusivity provides a quantitative measure
for evaluating how quickly a material will change temperature in comparison to its volumetric
heat capacity. Materials with a high thermal diffusivity will quickly change temperature. The
thermal diffusivities for quartz, alkali feldspar, and most other minerals and rocks are in the range
of approximately 1 × 10 –10 × 10 m /s. For comparison, the thermal diffusivities of many com-
2
–6
–6
–4
mon metals are in the range of 1 × 10 –5 × 10 m /s. Thus, a metal will heat up or cool down ten
2
–4