Page 85 - Geothermal Energy Renewable Energy and The Environment
P. 85
70 Geothermal Energy: Renewable Energy and the Environment
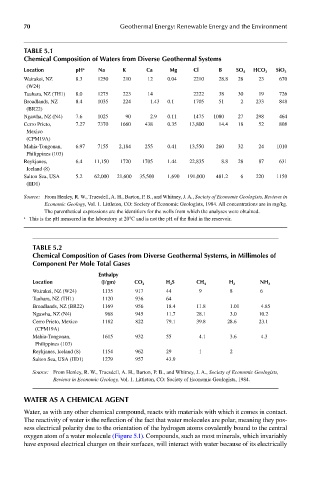
Table 5.1
chemical composition of waters from diverse Geothermal systems
location ph a na k ca mg cl b so 4 hco 3 sio 2
Wairakei, NZ 8.3 1250 210 12 0.04 2210 28.8 28 23 670
(W24)
Tauhara, NZ (TH1) 8.0 1275 223 14 2222 38 30 19 726
Broadlands, NZ 8.4 1035 224 1.43 0.1 1705 51 2 233 848
(BR22)
Ngawha, NZ (N4) 7.6 1025 90 2.9 0.11 1475 1080 27 298 464
Cerro Prieto, 7.27 7370 1660 438 0.35 13,800 14.4 18 52 808
Mexico
(CPM19A)
Mahia-Tongonan, 6.97 7155 2,184 255 0.41 13,550 260 32 24 1010
Philippines (103)
Reykjanes, 6.4 11,150 1720 1705 1.44 22,835 8.8 28 87 631
Iceland (8)
Salton Sea, USA 5.2 62,000 21,600 35,500 1,690 191,000 481.2 6 220 1150
(IID1)
Source: From Henley, R. W., Truesdell, A. H., Barton, P. B., and Whitney, J. A., Society of Economic Geologists, Reviews in
Economic Geology, Vol. 1. Littleton, CO: Society of Economic Geologists, 1984. All concentrations are in mg/kg.
The parenthetical expressions are the identifiers for the wells from which the analyses were obtained.
a This is the pH measured in the laboratory at 20°C and is not the pH of the fluid in the reservoir.
Table 5.2
chemical composition of Gases from diverse Geothermal systems, in millimoles of
component per mole Total Gases
enthalpy
location (J/gm) co 2 h 2 s ch 4 h 2 nh 3
Wairakei, NZ (W24) 1135 917 44 9 8 6
Tauhara, NZ (TH1) 1120 936 64
Broadlands, NZ (BR22) 1169 956 18.4 11.8 1.01 4.85
Ngawha, NZ (N4) 968 945 11.7 28.1 3.0 10.2
Cerro Prieto, Mexico 1182 822 79.1 39.8 28.6 23.1
(CPM19A)
Mahia-Tongonan, 1615 932 55 4.1 3.6 4.3
Philippines (103)
Reykjanes, Iceland (8) 1154 962 29 1 2
Salton Sea, USA (IID1) 1279 957 43.9
Source: From Henley, R. W., Truesdell, A. H., Barton, P. B., and Whitney, J. A., Society of Economic Geologists,
Reviews in Economic Geology, Vol. 1. Littleton, CO: Society of Economic Geologists, 1984.
waTer as a chemIcal aGenT
Water, as with any other chemical compound, reacts with materials with which it comes in contact.
The reactivity of water is the reflection of the fact that water molecules are polar, meaning they pos-
sess electrical polarity due to the orientation of the hydrogen atoms covalently bound to the central
oxygen atom of a water molecule (Figure 5.1). Compounds, such as most minerals, which invariably
have exposed electrical charges on their surfaces, will interact with water because of its electrically