Page 93 - Geothermal Energy Renewable Energy and The Environment
P. 93
78 Geothermal Energy: Renewable Energy and the Environment
2.0
1.8
1.6
Log K 1.4 KAlSi O + Na +
8
3
1.2
1.0
NaAlSi O + K +
8
3
0.8
0.6
0 50 100 150 200 250 300 350
T (°C)
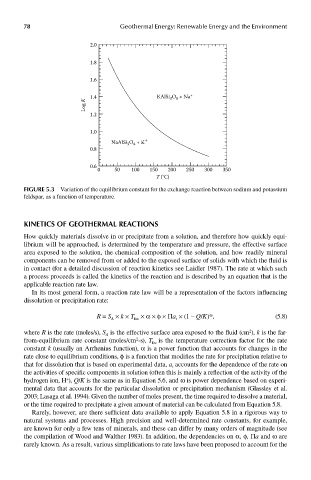
FIGUre 5.3 Variation of the equilibrium constant for the exchange reaction between sodium and potassium
feldspar, as a function of temperature.
kIneTIcs oF GeoThermal reacTIons
How quickly materials dissolve in or precipitate from a solution, and therefore how quickly equi-
librium will be approached, is determined by the temperature and pressure, the effective surface
area exposed to the solution, the chemical composition of the solution, and how readily mineral
components can be removed from or added to the exposed surface of solids with which the fluid is
in contact (for a detailed discussion of reaction kinetics see Laidler 1987). The rate at which such
a process proceeds is called the kinetics of the reaction and is described by an equation that is the
applicable reaction rate law.
In its most general form, a reaction rate law will be a representation of the factors influencing
dissolution or precipitation rate:
ω
R = S × k × T × α × ϕ × Πa × (1 – Q/K) , (5.8)
A
fac
i
2
where R is the rate (moles/s), S is the effective surface area exposed to the fluid (cm ), k is the far-
A
2
from-equilibrium rate constant (moles/cm -s), T is the temperature correction factor for the rate
fac
constant k (usually an Arrhenius function), α is a power function that accounts for changes in the
rate close to equilibrium conditions, ϕ is a function that modifies the rate for precipitation relative to
that for dissolution that is based on experimental data, a accounts for the dependence of the rate on
i
the activities of specific components in solution (often this is mainly a reflection of the activity of the
+
hydrogen ion, H ), Q/K is the same as in Equation 5.6, and ω is power dependence based on experi-
mental data that accounts for the particular dissolution or precipitation mechanism (Glassley et al.
2003; Lasaga et al. 1994). Given the number of moles present, the time required to dissolve a material,
or the time required to precipitate a given amount of material can be calculated from Equation 5.8.
Rarely, however, are there sufficient data available to apply Equation 5.8 in a rigorous way to
natural systems and processes. High precision and well-determined rate constants, for example,
are known for only a few tens of minerals, and these can differ by many orders of magnitude (see
the compilation of Wood and Walther 1983). In addition, the dependencies on α, ϕ, Πa and ω are
rarely known. As a result, various simplifications to rate laws have been proposed to account for the