Page 94 - Geothermal Energy Renewable Energy and The Environment
P. 94
Chemistry of Geothermal Fluids 79
behavior of particular minerals or suites of minerals for a limited range of mineral-fluid conditions
(e.g., Aagaard and Helgeson 1977; Chen and Brantley 1997; Lasaga 1981, 1986; Wood and Walther
1983; Velbel 1989), or simplifying assumptions are made to allow approximations to be computed
for the extent to which reaction progress occurs.
Nevertheless, substantial insight can be gained by considering how rates of reactions influence
what can be observed in natural systems, even using the limited information available to us. As an
example of the insight that can be gained by evaluating reaction progress, consider the question of
how reaction time is affected by grain size and temperature. Assume that we are considering a sys-
tem in which a mineral has a known dissolution rate constant that was experimentally measured for
a given set of conditions. We also know how that rate constant changes as temperature rises beyond
the conditions for which Q/K equals 1.0.
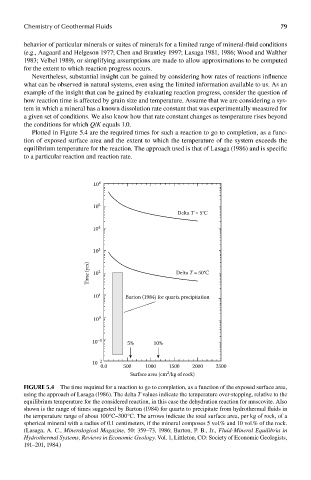
Plotted in Figure 5.4 are the required times for such a reaction to go to completion, as a func-
tion of exposed surface area and the extent to which the temperature of the system exceeds the
equilibrium temperature for the reaction. The approach used is that of Lasaga (1986) and is specific
to a particular reaction and reaction rate.
10 6
10 5
Delta T = 5°C
10 4
10 3
Time (yrs) 10 2 Delta T = 50°C
10 1 Barton (1984) for quartz precipitation
10 0
10 –1 5% 10%
10 –2
0.0 500 1000 1500 2000 2500
2
Surface area (cm /kg of rock)
FIGUre 5.4 The time required for a reaction to go to completion, as a function of the exposed surface area,
using the approach of Lasaga (1986). The delta T values indicate the temperature over-stepping, relative to the
equilibrium temperature for the considered reaction, in this case the dehydration reaction for muscovite. Also
shown is the range of times suggested by Barton (1984) for quartz to precipitate from hydrothermal fluids in
the temperature range of about 100°C–300°C. The arrows indicate the total surface area, per kg of rock, of a
spherical mineral with a radius of 0.1 centimeters, if the mineral composes 5 vol.% and 10 vol.% of the rock.
(Lasaga, A. C., Mineralogical Magazine, 50: 359–73, 1986; Barton, P. B., Jr., Fluid-Mineral Equilibria in
Hydrothermal Systems, Reviews in Economic Geology. Vol. 1, Littleton, CO: Society of Economic Geologists,
191–201, 1984.)