Page 96 - Geothermal Energy Renewable Energy and The Environment
P. 96
Chemistry of Geothermal Fluids 81
The influence of these components is expressed in the aqueous chemistry that occurs in solution.
Some of the reactions in which these compounds are involved, and their respective log K values at
25°C, are the following:
–
+
H S (aq) < = > H + HS log K = –6.9877
2
–
H SO < = > H + HSO log K = 1.0209
+
4
2
4
=
–
+
HSO < = > H + HSO log K = –1.9791
4
4
–
+
HCl (aq) < = > H + Cl log K = 0.67
–
+
CO (aq) + H O < = > H + HCO log K = –6.3447
3
2
2
–
=
+
HCO < = > H + CO log K = –10.3288
3
3
Notice that all of these reactions involve H+, which strongly influences the acidity of a solution (see
Sidebar for discussion of acidity).
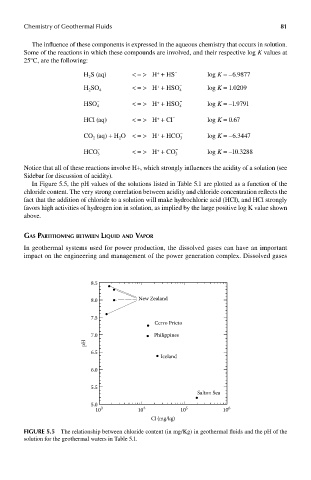
In Figure 5.5, the pH values of the solutions listed in Table 5.1 are plotted as a function of the
chloride content. The very strong correlation between acidity and chloride concentration reflects the
fact that the addition of chloride to a solution will make hydrochloric acid (HCl), and HCl strongly
favors high activities of hydrogen ion in solution, as implied by the large positive log K value shown
above.
Gas parTiTioninG beTween liquid and vapor
In geothermal systems used for power production, the dissolved gases can have an important
impact on the engineering and management of the power generation complex. Dissolved gases
8.5
8.0 New Zealand
7.5
Cerro Prieto
7.0 Philippines
pH
6.5
Iceland
6.0
5.5
Salton Sea
5.0
10 3 10 4 10 5 10 6
Cl (mg/kg)
FIGUre 5.5 The relationship between chloride content (in mg/Kg) in geothermal fluids and the pH of the
solution for the geothermal waters in Table 5.1.