Page 98 - Geothermal Energy Renewable Energy and The Environment
P. 98
Chemistry of Geothermal Fluids 83
Table 5.4
correlation parameters for use in calculating
distribution coefficients according to equation 5.9
solute e F G h
2286.4159 11.3397 −70.7279 63.0631
H 2
2305.0674 −11.3240 25.3224 −15.6449
O 2
1672.9376 28.1751 −112.4619 85.3807
CO 2
H 2 S 1319.1205 14.1571 −46.8361 33.2266
CH4 2215.6977 −0.1089 −6.6240 4.6789
Source: Data are from Fernandez-Prini, R., Alvarez, J. L., and Harvey, A. H.,
Aqueous Systems at Elevated Temperatures and Pressures: Physical
Chemistry in Water, Steam and Hydrothermal Solutions, London:
Elsevier, Ltd., 73–98, 2004.
7.0
6.0
O 2
5.0
H 2
Log Kd 4.0
3.0 CO 2
2.0
H S
2
1.0
0 50 100 150 200 250 300 350
Temperature (°C )
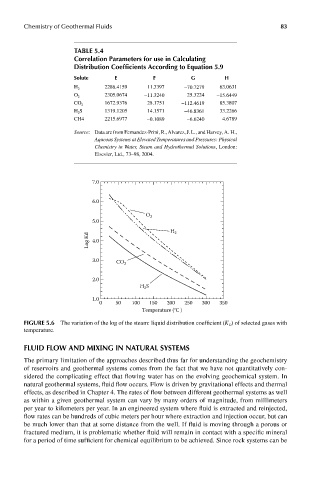
FIGUre 5.6 The variation of the log of the steam: liquid distribution coefficient (K D ) of selected gases with
temperature.
FlUId Flow and mIxInG In naTUral sysTems
The primary limitation of the approaches described thus far for understanding the geochemistry
of reservoirs and geothermal systems comes from the fact that we have not quantitatively con-
sidered the complicating effect that flowing water has on the evolving geochemical system. In
natural geothermal systems, fluid flow occurs. Flow is driven by gravitational effects and thermal
effects, as described in Chapter 4. The rates of flow between different geothermal systems as well
as within a given geothermal system can vary by many orders of magnitude, from millimeters
per year to kilometers per year. In an engineered system where fluid is extracted and reinjected,
flow rates can be hundreds of cubic meters per hour where extraction and injection occur, but can
be much lower than that at some distance from the well. If fluid is moving through a porous or
fractured medium, it is problematic whether fluid will remain in contact with a specific mineral
for a period of time sufficient for chemical equilibrium to be achieved. Since rock systems can be