Page 128 - Glucose Monitoring Devices
P. 128
Commercial systems 129
where the incision was made with the blunt dissector, which will lock into place.
Confirm the sensor is in place by lightly touch the insertion area before removing
the insertion tool from the incision. Close and correctly dress the incision with a
skin adhesive, such as Steri-Strip.
Summary
This chapter has presented an array of glucose transduction techniques and system
implementations that define the currently available commercial systems as well as
proved the building blocks for future development. The glucose oxidase and
fluorescence-based technologies have been built into systems that have been CE
marked FDA approved for sale in globally. As part of the regulatory approval pro-
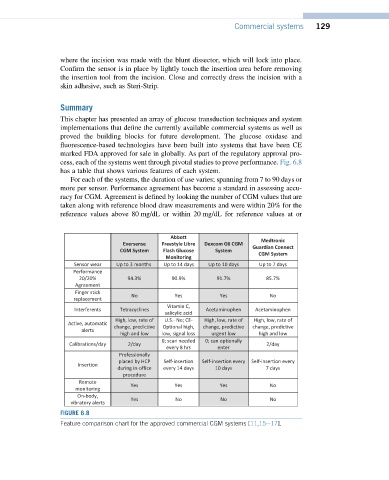
cess, each of the systems went through pivotal studies to prove performance. Fig. 6.8
has a table that shows various features of each system.
For each of the systems, the duration of use varies; spanning from 7 to 90 days or
more per sensor. Performance agreement has become a standard in assessing accu-
racy for CGM. Agreement is defined by looking the number of CGM values that are
taken along with reference blood draw measurements and were within 20% for the
reference values above 80 mg/dL or within 20 mg/dL for reference values at or
Abbott
Medtronic
Eversense Freestyle Libre Dexcom G6 CGM
Guardian Connect
CGM System Flash Glucose System
CGM System
Monitoring
Sensor wear Up to 3 months Up to 14 days Up to 10 days Up to 7 days
Performance
20/20% 94.3% 90.9% 91.7% 85.7%
Agreement
Finger stick
No Yes Yes No
replacement
Vitamin C,
Interferents Tetracyclines Acetaminophen Acetaminophen
salicylic acid
High, low, rate of U.S.- No; CE- High, low, rate of High, low, rate of
Active, automatic
change, predictive Optional high, change, predictive change, predictive
alerts
high and low low, signal loss urgent low high and low
0; scan needed 0; can optionally
Calibrations/day 2/day 2/day
every 8 hrs enter
Professionally
placed by HCP Self-insertion Self-insertion every Self-insertion every
Insertion
during in-office every 14 days 10 days 7 days
procedure
Remote
Yes Yes Yes No
monitoring
On-body,
Yes No No No
vibratory alerts
FIGURE 6.8
Feature comparison chart for the approved commercial CGM systems [11,15e17].