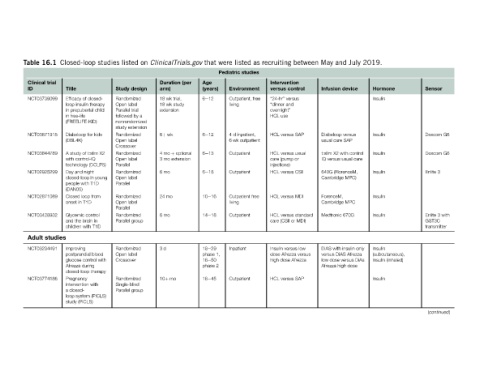
Page 334 - Glucose Monitoring Devices
P. 334
Sensor Dexcom G6 Dexcom G6 Enlite 3 Enlite 3 with GST3C transmitter (continued)
Hormone Insulin Insulin Insulin Insulin Insulin Insulin Insulin (subcutaneous), Insulin (inhaled) Insulin
2019.
July device
and Diabeloop versus usual care SAP t:slim X2 with control IQ versus usual care 640G (FlorenceM, Cambridge MPC) Cambridge MPC Medtronic 670G DiAS with insulin only versus DiAS Afrezza low dose versus DiAs Afrezza high dose
May Infusion ForenceM,
between control HCL versus standard
recruiting Intervention versus “24-hr” versus “dinner and overnight” HCL use HCL versus SAP HCL versus usual care (pump or injections) HCL versus CSII HCL versus MDI care (CSII or MDI) Insulin versus low dose Afrezza versus high dose Afrezza HCL versus SAP
as studies
listed Environment Outpatient, free living 4 d inpatient, 6 wk outpatient Outpatient Outpatient Outpatient free living Outpatient Inpatient Outpatient
were Pediatric
that Age (years) 6e12 6e12 6e13 6e18 10e16 14e18 18e29 phase 1, 18e50 phase 2 18e45
ClinicalTrials.gov (per Duration arm) 18 wk trial, 18 wk study extension 6þ wk 4mo þ optional 3 mo extension 6mo 24 mo 6mo 3d 10þ mo
on design
listed Study Randomized Open label Parallel trial followed by a nonrandomized study extension Randomized Open label Crossover Randomized Open label Parallel Randomized Open label Parallel Randomized Open label Parallel Randomized Parallel group Randomized Open label Crossover Randomized Single-blind Parallel group
studies
Closed-loop Title Efficacy of closed- loop insulin therapy in prepubertal child in free-life (FREELIFE-KID) Diabeloop for kids (DBL4K) A study of t:slim X2 with control-IQ technology (DCLP5) Day and night closed-loop in young people with T1D (DAN05) Closed loop from onset in T1D Glycemic control and the brain in children with T1D Improving postprandial blood glucose control with Afrezza
16.1 trial NCT03739099 NCT03671915 NCT03844789 NCT02925299 NCT02871089 NCT03428932 studies NCT03234491 NCT03774186
Table Clinical ID Adult