Page 338 - Glucose Monitoring Devices
P. 338
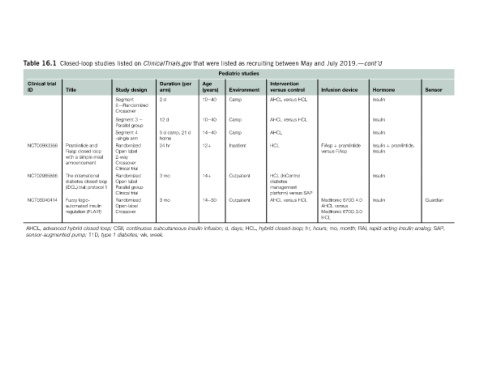
Sensor Guardian SAP,
analog; insulin
Hormone Insulin Insulin Insulin Insulin þ pramlintide, Insulin Insulin Insulin rapid-acting
2019.dcont’d device RAI, month;
July Infusion FiAsp þ pramlintide versus FiAsp Medtronic 670G 4.0 AHCL versus Medtronic 670G 3.0 HCL mo,
and hours;
May hr,
between Intervention control versus AHCL versus HCL AHCL versus HCL AHCL HCL HCL (InControl diabetes management platform) versus SAP AHCL versus HCL closed-loop;
recruiting hybrid HCL,
as studies Environment Camp Camp Camp Inpatient Outpatient Outpatient days;
listed Pediatric d,
were Age (years) 10e40 10e40 14e40 12þ 14þ 14e30 infusion;
that (per insulin
ClinicalTrials.gov Duration arm) 2d 12 d 5 d camp, 21 d home 24 hr 3mo 3mo subcutaneous week.
on design 2dRandomized continuous wk,
listed Study Segment Crossover Segment 3 e Parallel group Segment 4 -single arm Randomized Open label 2-way Crossover Clinical trial Randomized Open label Parallel group Clinical trial Randomized Open-label Crossover CSII, diabetes; 1
studies loop; closed type T1D,
Closed-loop Title Pramlintide and Fiasp closed loop with a simple meal announcement The international diabetes closed loop (iDCL) trial: protocol 1 Fuzzy logic- automated insulin regulation (FLAIR) hybrid pump;
16.1 trial NCT03993366 NCT02985866 NCT03040414 advanced sensor-augmented
Table Clinical ID AHCL,