Page 336 - Glucose Monitoring Devices
P. 336
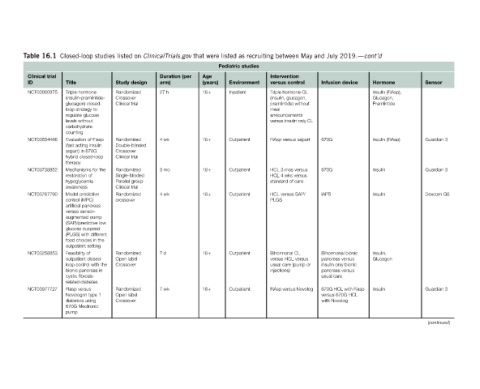
Sensor Guardian 3 Guardian 3 Dexcom G6 Guardian 3 (continued)
Hormone Insulin (FiAsp), Glucagon, Pramlintide Insulin (FiAsp) Insulin Insulin Insulin, Glucagon Insulin
2019.dcont’d device
July Infusion 670G 670G iAPS Bihormonal bionic pancreas versus insulin only bionic pancreas versus usual care 670G HCL with Fiasp versus 670G HCL with Novolog
and
May
between Intervention control versus Triple hormone CL (insulin, glucagon, pramlintide) without meal announcements versus insulin only CL FiAsp versus aspart HCL 3 mos versus HCL 4 wks versus standard of care HCL versus SAP/ PLGS Bihormonal CL versus HCL versus usual care (pump or injections) FiAsp versus Novolog
recruiting
as studies Environment Inpatient Outpatient Outpatient Outpatient Outpatient Outpatient
listed Pediatric
were Age (years) 18þ 18þ 18þ 18þ 18þ 18þ
that (per
ClinicalTrials.gov Duration arm) 27 h 4wk 3mo 4wk 7d 7wk
on design
listed Study Randomized Crossover Clinical trial Randomized Double-blinded Crossover Clinical trial Randomized Single-blinded Parallel group Clinical trial Randomized crossover Randomized Open label Crossover Randomized Open label Crossover
studies
Closed-loop Title Triple-hormone (insulin-pramlintide- glucagon) closed- loop strategy to regulate glucose levels without carbohydrate counting Evaluation of Fiasp (fast acting insulin aspart) in 670G hybrid closed-loop therapy Mechanisms for the restoration of hypoglycemia awareness Model predictive control (MPC) artificial pancreas versus sensor- augmented pump (SAP)/predictive low gluc
16.1 trial NCT03800875 NCT03554486 NCT03738852 NCT03767790 NCT03258853 NCT03977727
Table Clinical ID