Page 337 - Glucose Monitoring Devices
P. 337
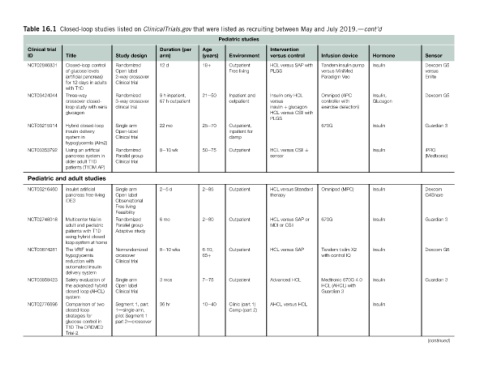
Sensor Dexcom G5 versus Enlite Dexcom G5 Guardian 3 iPRO (Medtronic) Dexcom G4Share Guardian 3 Dexcom G6 Guardian 3 (continued)
Hormone Insulin Insulin, Glucagon Insulin Insulin Insulin Insulin Insulin Insulin Insulin
2019.dcont’d device
July Infusion Tandem insulin pump versus MiniMed Paradigm Veo Omnipod (APC controller with exercise detection) 670G Omnipod (MPC) 670G Tandem t:slim X2 with control IQ Medtronic 670G 4.0 HCL (AHCL) with Guardian 3
and
May
between Intervention control versus HCL versus SAP with PLGS Insulin only HCL versus insulin þ glucagon HCL versus CSII with PLGS HCL versus CSII sensor HCL versus Standard therapy HCL versus SAP or MDI or CSII HCL versus SAP Advanced HCL AHCL versus HCL
recruiting
as studies Environment Outpatient Free living Inpatient and outpatient Outpatient, inpatient for clamp Outpatient Outpatient Outpatient Outpatient Outpatient Clinic (part 1) Camp (part 2)
listed Pediatric
were Age (years) 18þ 21e50 25e70 50e75 2e85 2e80 6-10, 65þ 7e75 10e40
that (per
ClinicalTrials.gov Duration arm) 12 d 9 h inpatient, 67 h outpatient 22 mo 8e10 wk 2e5d 6mo 8e10 wks 3 mos 36 hr
on design 2-way crossover 3-way crossover Nonrandomized Segment 1, part pilot Segment 1 part 2dcrossover
listed Study Randomized Open label Clinical trial Randomized clinical trial Single arm Open-label Clinical trial Randomized Parallel group Clinical trial Single arm Open label Observational Free living Feasibility Randomized Parallel group Adaptive study crossover Clinical trial Single arm Open label Clinical trial 1dsingle arm,
studies studies
Closed-loop Title Closed-loop control of glucose levels (artificial pancreas) for 12 days in adults with T1D Three-way crossover closed- loop study with xeris glucagon Hybrid closed-loop insulin delivery system in hypoglycemia (Aim2) Using an artificial pancreas system in older adult T1D patients (T1DM AP) adult Insulet artificial pancreas free-living IDE3 Multicenter trial in adult and pediatric
16.1 trial NCT02846831 NCT03424044 NCT03215914 NCT03353792 and Pediatric NCT03216460 NCT02748018 NCT03674281 NCT03959423 NCT02776696
Table Clinical ID