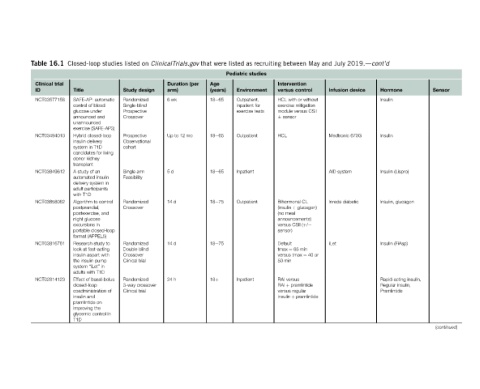
Page 335 - Glucose Monitoring Devices
P. 335
Sensor (continued)
Hormone Insulin Insulin Insulin (Lispro) Insulin, glucagon Insulin (FiAsp) Rapid-acting insulin, Regular insulin, Pramlintide
2019.dcont’d device
July Infusion Medtronic 670G AID system Inreda diabetic
and iLet
May
between Intervention control versus HCL with or without exercise mitigation module versus CSII sensor HCL Bihormonal CL (insulin þ glucagon) (no meal announcements) versus CSII (þ/ sensor) Default tmax ¼ 65 min versus tmax ¼ 40 or 50 min RAI versus RAI þ pramlintide versus regular insulin þ pramlintide
recruiting
as studies Environment Outpatient, inpatient for exercise tests Outpatient Inpatient Outpatient Inpatient
listed Pediatric
were Age (years) 18e65 18e65 18e65 18e75 18e75 18þ
that (per
ClinicalTrials.gov Duration arm) 6wk Up to 12 mo 5d 14 d 14 d 24 h
on design 3-way crossover
listed Study Randomized Single-blind Prospective Crossover Prospective Observational cohort Single arm Feasibility Randomized Crossover Randomized Double blind Crossover Clinical trial Randomized Clinical trial
studies
Closed-loop Title SAFE-AP: automatic control of blood glucose under announced and unannounced exercise (SAFE-AP3) Hybrid closed-loop insulin delivery system in T1D candidates for living donor kidney transplant A study of an automated insulin delivery system in adult participants with T1D Algorithm to control postprandial, postexercise, and night glucose excursions in portable closed-loo
16.1 trial NCT03577158 NCT03494010 NCT03849612 NCT03858062 NCT03816761 NCT02814123
Table Clinical ID