Page 340 - Handbook of Thermal Analysis of Construction Materials
P. 340
Section 4.0 - Slags 317
increase in the amount of the C-S-H phase. In certain mixes containing
larger amounts of Ca(OH) , the formation of α-C SH resulted in low
2
2
strengths. The CH, C-S-H, and α-C SH (endothermal peak at 500°C)
2
phases could be identified by DTA.
The long-term behavior of slag concrete exposed to carbonation is
a subject of controversy. Thermal methods are suitable to monitor the depth
of carbonation by estimating CaCO , as well as the Ca(OH) contents. In a
3
2
systematic study, Litvan and Meyer [48] examined core samples taken from
different depths from slag concrete walls that were exposed to natural
elements for 20 years. The amounts of carbonation and lime at various
depths were determined by TG from the inside and outside of the building.
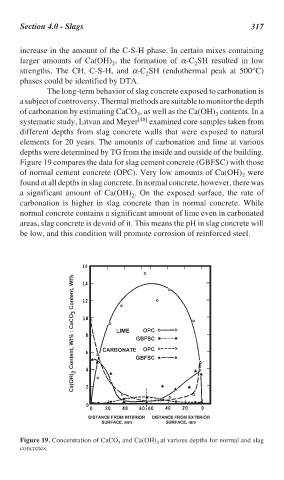
Figure 19 compares the data for slag cement concrete (GBFSC) with those
of normal cement concrete (OPC). Very low amounts of Ca(OH) were
2
found at all depths in slag concrete. In normal concrete, however, there was
a significant amount of Ca(OH) . On the exposed surface, the rate of
2
carbonation is higher in slag concrete than in normal concrete. While
normal concrete contains a significant amount of lime even in carbonated
areas, slag concrete is devoid of it. This means the pH in slag concrete will
be low, and this condition will promote corrosion of reinforced steel.
Figure 19. Concentration of CaCO and Ca(OH) at various depths for normal and slag
3
2
concretes.