Page 505 - Handbook of Thermal Analysis of Construction Materials
P. 505
Section 8.0 - A Three Step Gypsum Dehydration Process 477
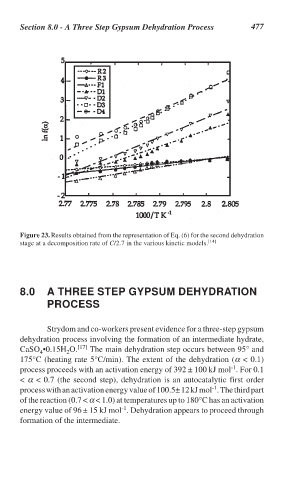
Figure 23. Results obtained from the representation of Eq. (6) for the second dehydration
stage at a decomposition rate of C/2.7 in the various kinetic models. [14]
8.0 A THREE STEP GYPSUM DEHYDRATION
PROCESS
Strydom and co-workers present evidence for a three-step gypsum
dehydration process involving the formation of an intermediate hydrate,
CaSO •0.15H O. [17] The main dehydration step occurs between 95° and
2
4
175°C (heating rate 5°C/min). The extent of the dehydration (α < 0.1)
-1
process proceeds with an activation energy of 392 ± 100 kJ mol . For 0.1
< α < 0.7 (the second step), dehydration is an autocatalytic first order
-1
process with an activation energy value of 100.5 ± 12 kJ mol . The third part
of the reaction (0.7 < α < 1.0) at temperatures up to 180°C has an activation
-1
energy value of 96 ± 15 kJ mol . Dehydration appears to proceed through
formation of the intermediate.