Page 507 - Handbook of Thermal Analysis of Construction Materials
P. 507
Section 8.0 - A Three Step Gypsum Dehydration Process 479
Eq. (8) CaSO •0.5H O → CaSO •0.15H O + 0.35H O
4
2
4
2
2
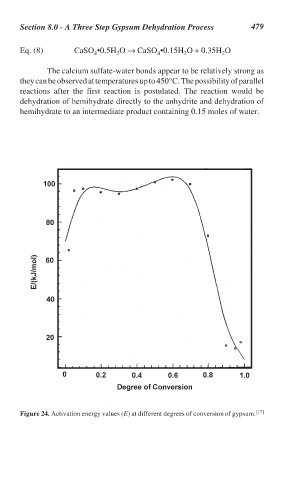
The calcium sulfate-water bonds appear to be relatively strong as
they can be observed at temperatures up to 450°C. The possibility of parallel
reactions after the first reaction is postulated. The reaction would be
dehydration of hemihydrate directly to the anhydrite and dehydration of
hemihydrate to an intermediate product containing 0.15 moles of water.
Figure 24. Activation energy values (E) at different degrees of conversion of gypsum. [17]