Page 273 - Geology and Geochemistry of Oil and Gas
P. 273
236 MATHEMATICAL MODELING IN PETROLEUM GEOLOGY
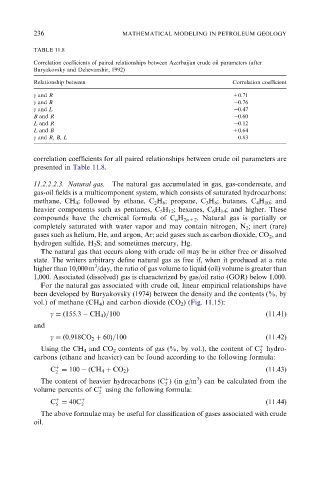
TABLE 11.8
Correlation coefficients of paired relationships between Azerbaijan crude oil parameters (after
Buryakovsky and Dzhevanshir, 1992)
Relationship between Correlation coefficient
g and R +0.71
g and B 0.76
g and L 0.47
B and R 0.60
L and R 0.12
L and B +0.64
g and R, B, L 0.83
correlation coefficients for all paired relationships between crude oil parameters are
presented in Table 11.8.
11.2.2.2.3. Natural gas. The natural gas accumulated in gas, gas-condensate, and
gas-oil fields is a multicomponent system, which consists of saturated hydrocarbons:
methane, CH 4 ; followed by ethane, C 2 H 6 ; propane, C 3 H 8 ; butanes, C 4 H 10 ; and
heavier components such as pentanes, C 5 H 12 ; hexanes, C 6 H 14 ; and higher. These
compounds have the chemical formula of C n H 2n+2 . Natural gas is partially or
completely saturated with water vapor and may contain nitrogen, N 2 ; inert (rare)
gases such as helium, He, and argon, Ar; acid gases such as carbon dioxide, CO 2 , and
hydrogen sulfide, H 2 S; and sometimes mercury, Hg.
The natural gas that occurs along with crude oil may be in either free or dissolved
state. The writers arbitrary define natural gas as free if, when it produced at a rate
3
higher than 10,000 m /day, the ratio of gas volume to liquid (oil) volume is greater than
1,000. Associated (dissolved) gas is characterized by gas/oil ratio (GOR) below 1,000.
For the natural gas associated with crude oil, linear empirical relationships have
been developed by Buryakovsky (1974) between the density and the contents (%, by
vol.) of methane (CH 4 ) and carbon dioxide (CO 2 ) (Fig. 11.15):
g ¼ ð155:3 CH 4 Þ=100 (11.41)
and
g ¼ ð0:918CO 2 þ 60Þ=100 (11.42)
þ
Using the CH 4 and CO 2 contents of gas (%, by vol.), the content of C hydro-
2
carbons (ethane and heavier) can be found according to the following formula:
þ
C ¼ 100 ðCH 4 þ CO 2 Þ (11.43)
2
þ
3
The content of heavier hydrocarbons (C ) (in g/m ) can be calculated from the
5
þ
volume percents of C using the following formula:
2
þ
C ¼ 40C þ (11.44)
5 2
The above formulae may be useful for classification of gases associated with crude
oil.