Page 245 - Handbook Of Multiphase Flow Assurance
P. 245
244 10. Research methods in flow assurance
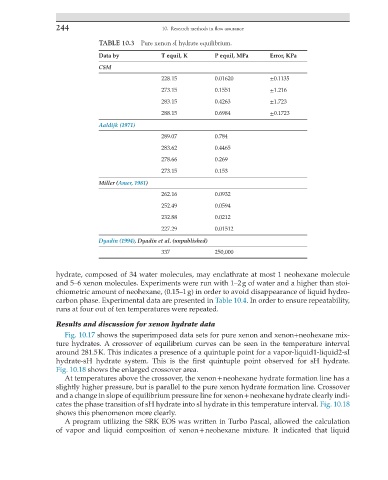
TABLE 10.3 Pure xenon sI hydrate equilibrium.
Data by T equil, K P equil, MPa Error, KPa
CSM
228.15 0.01620 ±0.1135
273.15 0.1551 ±1.216
283.15 0.4263 ±1.723
288.15 0.6984 ±0.1723
Aaldijk (1971)
289.07 0.784
283.62 0.4465
278.66 0.269
273.15 0.153
Miller (Amer, 1981)
262.16 0.0932
252.49 0.0594
232.88 0.0212
227.29 0.01512
Dyadin (1994), Dyadin et al. (unpublished)
337 250,000
hydrate, composed of 34 water molecules, may enclathrate at most 1 neohexane molecule
and 5–6 xenon molecules. Experiments were run with 1–2 g of water and a higher than stoi-
chiometric amount of neohexane, (0.15–1 g) in order to avoid disappearance of liquid hydro-
carbon phase. Experimental data are presented in Table 10.4. In order to ensure repeatability,
runs at four out of ten temperatures were repeated.
Results and discussion for xenon hydrate data
Fig. 10.17 shows the superimposed data sets for pure xenon and xenon+neohexane mix-
ture hydrates. A crossover of equilibrium curves can be seen in the temperature interval
around 281.5 K. This indicates a presence of a quintuple point for a vapor-liquid1- liquid2-sI
hydrate-sH hydrate system. This is the first quintuple point observed for sH hydrate.
Fig. 10.18 shows the enlarged crossover area.
At temperatures above the crossover, the xenon + neohexane hydrate formation line has a
slightly higher pressure, but is parallel to the pure xenon hydrate formation line. Crossover
and a change in slope of equilibrium pressure line for xenon + neohexane hydrate clearly indi-
cates the phase transition of sH hydrate into sI hydrate in this temperature interval. Fig. 10.18
shows this phenomenon more clearly.
A program utilizing the SRK EOS was written in Turbo Pascal, allowed the calculation
of vapor and liquid composition of xenon + neohexane mixture. It indicated that liquid