Page 246 - Handbook Of Multiphase Flow Assurance
P. 246
Hydrate stability and crystal growth 245
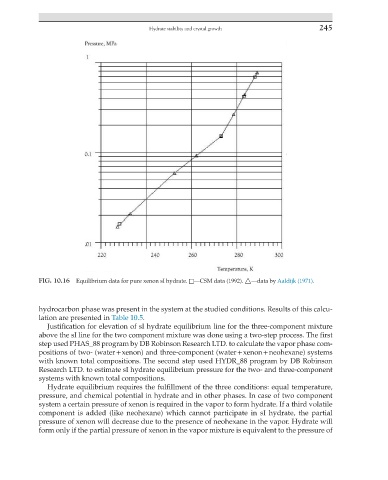
FIG. 10.16 Equilibrium data for pure xenon sI hydrate. □—CSM data (1992). △—data by Aaldijk (1971).
hydrocarbon phase was present in the system at the studied conditions. Results of this calcu-
lation are presented in Table 10.5.
Justification for elevation of sI hydrate equilibrium line for the three-component mixture
above the sI line for the two component mixture was done using a two-step process. The first
step used PHAS_88 program by DB Robinson Research LTD. to calculate the vapor phase com-
positions of two- (water + xenon) and three-component (water + xenon + neohexane) systems
with known total compositions. The second step used HYDR_88 program by DB Robinson
Research LTD. to estimate sI hydrate equilibrium pressure for the two- and three-component
systems with known total compositions.
Hydrate equilibrium requires the fulfillment of the three conditions: equal temperature,
pressure, and chemical potential in hydrate and in other phases. In case of two component
system a certain pressure of xenon is required in the vapor to form hydrate. If a third volatile
component is added (like neohexane) which cannot participate in sI hydrate, the partial
pressure of xenon will decrease due to the presence of neohexane in the vapor. Hydrate will
form only if the partial pressure of xenon in the vapor mixture is equivalent to the pressure of