Page 80 - Handbook of Adhesion Promoters
P. 80
4.2 Surface treatment 73
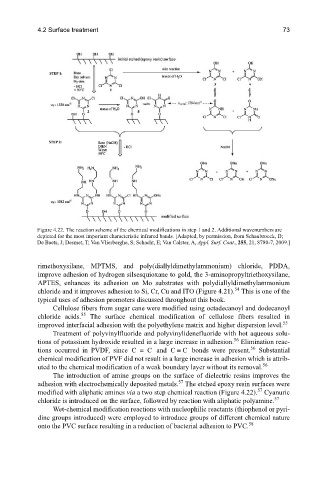
Figure 4.22. The reaction scheme of the chemical modifications in step 1 and 2. Additional wavenumbers are
depicted for the most important characteristic infrared bands. [Adapted, by permission, from Schaubroeck, D;
De Baets, J; Desmet, T; Van Vlierberghe, S; Schacht, E; Van Calster, A, Appl. Surf. Coat., 255, 21, 8780-7, 2009.]
rimethoxysilane, MPTMS, and poly(diallyldimethylammonium) chloride, PDDA,
improve adhesion of hydrogen silsesquioxane to gold, the 3-aminopropyltriethoxysilane,
APTES, enhances its adhesion on Mo substrates with polydiallyldimethylammonium
54
chloride and it improves adhesion to Si, Cr, Cu and ITO (Figure 4.21). This is one of the
typical uses of adhesion promoters discussed throughout this book.
Cellulose fibers from sugar cane were modified using octadecanoyl and dodecanoyl
55
chloride acids. The surface chemical modification of cellulose fibers resulted in
55
improved interfacial adhesion with the polyethylene matrix and higher dispersion level.
Treatment of polyvinylfluoride and polyvinylidenefluoride with hot aqueous solu-
56
tions of potassium hydroxide resulted in a large increase in adhesion. Elimination reac-
56
tions occurred in PVDF, since C C = and C C ≡ bonds were present. Substantial
chemical modification of PVF did not result in a large increase in adhesion which is attrib-
56
uted to the chemical modification of a weak boundary layer without its removal.
The introduction of amine groups on the surface of dielectric resins improves the
57
adhesion with electrochemically deposited metals. The etched epoxy resin surfaces were
57
modified with aliphatic amines via a two step chemical reaction (Figure 4.22). Cyanuric
57
chloride is introduced on the surface, followed by reaction with aliphatic polyamine.
Wet-chemical modification reactions with nucleophilic reactants (thiophenol or pyri-
dine groups introduced) were employed to introduce groups of different chemical nature
58
onto the PVC surface resulting in a reduction of bacterial adhesion to PVC.