Page 176 - Handbook of Battery Materials
P. 176
4.4 Conversion of EMD to LiMnO 2 or LiMn 2 O 4 for Rechargeable Li Batteries 145
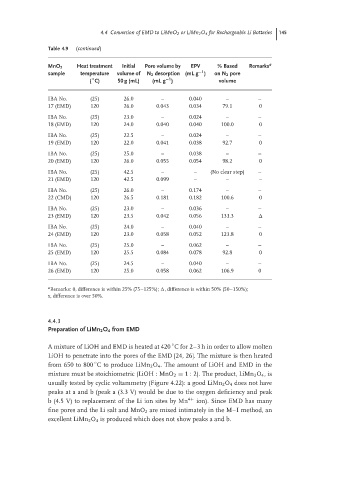
Table 4.9 (continued)
MnO 2 Heat treatment Initial Pore volume by EPV % Based Remarks a
−1
sample temperature volume of N 2 desorption (mL g ) on N 2 pore
◦
−1
( C) 50 g (mL) (mL g ) volume
IBA No. (25) 26.0 – 0.040 – –
17 (EMD) 120 26.0 0.043 0.034 79.1 0
IBA No. (25) 23.0 – 0.024 – –
18 (EMD) 120 24.0 0.040 0.040 100.0 0
IBA No. (25) 22.5 – 0.024 – –
19 (EMD) 120 22.0 0.041 0.038 92.7 0
IBA No. (25) 25.0 – 0.038 – –
20 (EMD) 120 26.0 0.055 0.054 98.2 0
IBA No. (25) 42.5 – – (No clear step) –
21 (EMD) 120 42.5 0.099 – – –
IBA No. (25) 26.0 – 0.174 – –
22 (CMD) 120 26.5 0.181 0.182 100.6 0
IBA No. (25) 23.0 – 0.036 – –
23 (EMD) 120 23.5 0.042 0.056 133.3
IBA No. (25) 24.0 – 0.040 – –
24 (EMD) 120 23.0 0.058 0.052 123.8 0
IBA No. (25) 25.0 – 0.062 – –
25 (EMD) 120 25.5 0.084 0.078 92.8 0
IBA No. (25) 24.5 – 0.040 – –
26 (EMD) 120 25.0 0.058 0.062 106.9 0
a Remarks: 0, difference is within 25% (75–125%); , difference is within 50% (50–150%);
x, difference is over 50%.
4.4.3
Preparation of LiMn 2 O 4 from EMD
◦
A mixture of LiOH and EMD is heated at 420 C for 2–3 h in order to allow molten
LiOH to penetrate into the pores of the EMD [24, 26]. The mixture is then heated
◦
from 650 to 800 Ctoproduce LiMn 2 O 4 . The amount of LiOH and EMD in the
mixture must be stoichiometric (LiOH : MnO 2 = 1 : 2). The product, LiMn 2 O 4 ,is
usually tested by cyclic voltammetry (Figure 4.22): a good LiMn 2 O 4 does not have
peaks at a and b (peak a (3.3 V) would be due to the oxygen deficiency and peak
b (4.5 V) to replacement of the Li ion sites by Mn 4+ ion). Since EMD has many
fine pores and the Li salt and MnO 2 are mixed intimately in the M–I method, an
excellent LiMn 2 O 4 is produced which does not show peaks a and b.