Page 174 - Handbook of Battery Materials
P. 174
4.4 Conversion of EMD to LiMnO 2 or LiMn 2 O 4 for Rechargeable Li Batteries 143
(1) + Water + Water
(c)
(a) (b)
EPV (cc)
(3) H
Water added
(a) (b) (c)
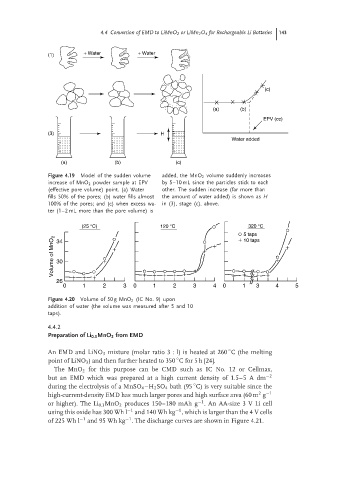
Figure 4.19 Model of the sudden volume added, the MnO 2 volume suddenly increases
increase of MnO 2 powder sample at EPV by 5–10 mL since the particles stick to each
(effective pore volume) point. (a) Water other. The sudden increase (far more than
fills 50% of the pores; (b) water fills almost the amount of water added) is shown as H
100% of the pores; and (c) when excess wa- in (3), stage (c), above.
ter (1–2 mL more than the pore volume) is
(25 °C) 120 °C 320 °C
5 taps
Volume of MnO 2 30
10 taps
34
26
0 1 2 3 0 1 2 3 4 0 1 3 4 5
Figure 4.20 Volume of 50 g MnO 2 (IC No. 9) upon
addition of water (the volume was measured after 5 and 10
taps).
4.4.2
Preparation of Li 0.3 MnO 2 from EMD
◦
An EMD and LiNO 3 mixture (molar ratio 3 : l) is heated at 260 C (the melting
◦
point of LiNO 3 ) and then further heated to 350 C for 5 h [24].
The MnO 2 for this purpose can be CMD such as IC No. 12 or Cellmax,
but an EMD which was prepared at a high current density of 1.5–5 A dm −2
during the electrolysis of a MnSO 4 –H 2 SO 4 bath (95 C) is very suitable since the
◦
2
high-current-density EMD has much larger pores and high surface area (60 m g −1
−1
or higher). The Li 0.3 MnO 2 produces 150–180 mAh g . An AA-size 3 V Li cell
−1
using this oxide has 300 Wh l −1 and 140 Wh kg , which is larger than the 4 V cells
−1
of 225 Wh l −1 and 95 Wh kg . The discharge curves are shown in Figure 4.21.