Page 173 - Handbook of Battery Materials
P. 173
142 4 Electrochemistry of Manganese Oxides
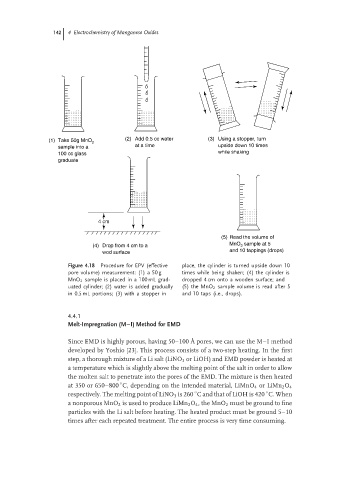
(1) Take 50g MnO 2 (2) Add 0.5 cc water (3) Using a stopper, turn
sample into a at a time upside down 10 times
while shaking
100 cc glass
graduate
4 cm
(5) Read the volume of
MnO sample at 5
(4) Drop from 4 cm to a 2
wod surface and 10 tappings (drops)
Figure 4.18 Procedure for EPV (effective place, the cylinder is turned upside down 10
pore volume) measurement: (1) a 50 g times while being shaken; (4) the cylinder is
MnO 2 sample is placed in a 100 mL grad- dropped 4 cm onto a wooden surface; and
uated cylinder; (2) water is added gradually (5) the MnO 2 sample volume is read after 5
in 0.5 mL portions; (3) with a stopper in and 10 taps (i.e., drops).
4.4.1
Melt-Impregnation (M–I) Method for EMD
Since EMD is highly porous, having 50–100 ˚ A pores, we can use the M–I method
developed by Yoshio [23]. This process consists of a two-step heating. In the first
step, a thorough mixture of a Li salt (LiNO 3 or LiOH) and EMD powder is heated at
a temperature which is slightly above the melting point of the salt in order to allow
the molten salt to penetrate into the pores of the EMD. The mixture is then heated
◦
at 350 or 650–800 C, depending on the intended material, LiMnO 4 or LiMn 2 O 4
respectively. The melting point of LiNO 3 is 260 C and that of LiOH is 420 C. When
◦
◦
a nonporous MnO 2 is used to produce LiMn 2 O 4 ,the MnO 2 must be ground to fine
particles with the Li salt before heating. The heated product must be ground 5–10
times after each repeated treatment. The entire process is very time consuming.